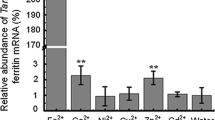
Abstract
The iron-storage protein ferritin has been purified to homogeneity from maize seeds, allowing to determine the sequence of the first 29 NH2-terminal amino acids of its subunit and to raise specific rabbit polyclonal antibodies. Addition of 500 μM Fe-EDTA/75 μM Fe-citrate to hydroponic culture solutions of maize plantlets, previously starved for iron, led to a significant increase of the iron concentration of roots and leaves, albeit root iron was mainly found associated with the apoplast. Immunodetection of ferritin by western blots indicated that this iron treatment induced ferritin protein accumulation in roots and leaves over a period of 3 days. In order to investigate this induction at the ferritin mRNA level, various ferritin cDNA clones were isolated from a cDNA library prepared from poly(A)+ mRNA isolated from roots 48 h after iron treatment. These cDNAs were classified into two groups called FM1 and FM2. Upstream of the sequence encoding the mature ferritin subunit, both of these cDNAs contained an in-frame coding sequence with the characteristics of a transit peptide for plastid targeting. Two members of the FM1 subfamily, both partial at their 5′ extremity, were characterized. They are identical, except in their 3′ untranslated region: FM1A extends 162 nucleotides beyond the 3′ terminus of FM1B. These two mRNAs could arise from the use of two different polyadenylation signals. FM2 is 96% identical to FM1 and contains 45 nucleotides of 5′ untranslated region. Northern analyses of root and leaf RNAs, at different times after iron treatment, revealed ferritin mRNA accumulation in response to iron. Ferritin mRNA accumulation was transient and particularly abundant in leaves, reaching a maximum at 24 h. The level of ferritin mRNA in roots was affected to a lesser extent than in leaves.
Similar content being viewed by others
References
Aziz N, Munro HN: Iron regulates ferritin mRNA translation through a segment of its untranslated region. Proc Natl Acad Sci USA: 84: 8478–8482 (1987).
Briat JF, Massenet O, Laulhère JP. A Jack Bean protein similar to pea seed ferritin and unrelated to concanavalin A is the only iron storage protein in Jack Bean seeds, although concanavalin A can form ferritin-like iron cores in vitro. J Biol Chem 264: 11550–11553 (1989).
Boyd D, Vecoli C, Belcher DM, Jain SK, Drysdale JW: Structural and functional relationships of human ferritin H and L chains deduced from cDNA clones. J Biol Chem 260: 11755–11761 (1985).
Cairo G, Bardella L, Schiaffonati L, Arosio P, Levi S, Bernelli-Zazzera A: Multiple mechanisms of iron-induced ferritin synthesis in HeLa cells. Biochem Biophys Res Comm 133: 314–321 (1985).
Crichton RR, Ponce-Ortiz Y, Koch MHJ, Parfait R, Stuhrmann HB: Isolation and characterization of phytoferritin from pea and lentil. Biochem J 171: 349–356 (1978).
Crichton RR. Proteins of iron storage and transport. Adv Protein Chem 40: 281–353 (1990).
Dickey LF, Sreedharan S, Theil EC, Didsbury JR, Wang YH, Kaufman RE: Differences in the regulation of messenger RNA from housekeeping and specialized-cell ferritin. J Biol Chem 262: 7901–7907 (1987).
Dickey LF, Wang YH, Shull GE, Wortman IA, Theil EC: The importance of the 3′-untranslated region in the translational control of ferritin mRNA. J Biol Chem 263: 3071–3074 (1988).
Drysdale JW, Munro HN: Regulation of synthesis and ferritin turnover of ferritin in rat liver. J Biol Chem 241: 3630–3637 (1966).
Fürst P, Hu S, Hackett R, Hamer D: Copper activates methalothionein gene transcription by altering the conformation of a specific DNA binding protein. Cell 55: 705–717 (1988).
Granick S: Protein apoferritin and ferritin in iron feeding and absorption. Science 103: 107 (1946).
Harrison PM, Artymiuk PJ, Ford GC, Lawson DM, Smith JMA, Treffry A, White JL: Ferritin: function and structural design of an iron-storage protein. In: Mann S, Webb J, Williams RJP (eds) Biomineralization: Chemical and Biomedical perspectives, pp. 257–294. VCN, Weinheim (1989).
Heijne GV, Steppuhn J, Herrmann RG: Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem 180: 535–545 (1989).
Hentze MW, Rouault TA, Caughman SW, Dancis A, Harford JB, Klausner RD: A cis-acting element is necessary and sufficient for translational regulation of human ferritin expression in response to iron. Proc Natl Acad Sci USA 84: 6730–6734 (1987).
Heusterspreute M, Crichton RR: Amino acid sequence of horse spleen apoferritin. FEBS Lett 129: 322–327 (1981).
Klausner RD, Harford JB: Cis-trans models for post-transcriptional gene regulation. Science 246: 870–872 (1989).
Koeller DM, Casey JL, Hentze MW, Gerhardt EM, Chan LN, Klausner RD, Harford JB: A cytosolic protein binds to structural elements within the iron regulatory region of the transferrin receptor mRNA. Proc Natl Acad Sci USA 86: 3574–3578 (1989).
Laulhère JP, Lescure AM, Briat JF: Purification and characterization of ferritins from maize, pea and soyabean seeds. J Biol Chem 263: 10289–10294 (1988).
Laulhère JP, Labouré AM, Briat JF: Mechanism of the transition from plant ferritin to phytosiderin. J Biol Chem 264: 3629–3635 (1989).
Laulhère JP, Labouré AM, van Wuytswinkel O, Gagnon J, Briat JF: Purification, characterization and function of bacterioferritin from the cyanobacteria Synechocystis PCC 6803. Biochem J (in press).
Leibold EA, Munro HN: Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5′ untranslated region of ferritin heavy- and light-subunit mRNAs. Proc Natl Acad Sci USA 85: 2171–2175 (1988).
Lescure AM, Proudhon D, Pesey H, Ragland M, Theil EC, Briat JF. Ferritin gene transcription is regulated by iron in soybean cell cultures. Proc Natl Acad Sci USA, 1991.
Lobreaux S, Briat JF: Ferritin accumulation and degradation in different organs of pea (Pisum sativum) during development. Biochem J 274: 601–606 (1991).
Logeman J, Shell J, Willmitzer L: Improved method for the isolation of RNA from plant tissues. Anal Biochem 163: 16–20 (1987).
Longnecker N, Welch RM: Accumulation of apoplastic iron in plant roots. Plant Physiol 92: 17–22 (1990).
Martin W, Lagrange T, Li YF, Bizance-Seyer C, Mache R. Hypothesis for the evolutionary origin of the chloroplast ribosomal protein L21 of spinach. Curr Genet 18: 553–556 (1991).
Mullner EW, Kuhn L: A stem loop in the 3′ untranslated region mediates iron-dependent regulation of transferrin receptor mRNA stability in the cytoplasm. Cell 53: 815–825 (1988).
Proudhon D, Briat JF, Lescure AM: Iron induction of ferritin synthesis in soybean cell suspensions. Plant Physiol 90: 586–590 (1989).
Ragland M, Briat JF, Gagnon J, Laulhère JP, Massenet O, Theil EC: Evidence for a conservation of ferritin sequences among plants and animals and for a transit peptide in soybean. J Biol Chem 265: 18339–18344 (1990).
Rosen KM, Villa-Kermaroff L: An alternative method for the visualisation of RNA in formaldehyde agarose gels. Focus 2: 23–24 (1990).
Rothenberger S, Müllner EW, Kühn LC: The mRNA-binding protein which controls ferritin and transferrin receptor expression is conserved during evolution. Nucl Acids Res 18: 1175–1179 (1990).
Rouault TA, Hentze MW, Haile DJ, Harford JB, Klausner RD: The iron-responsive element binding protein: a method for the affinity purification of a regulatory RNA-binding protein. Proc Natl Acad Sci USA 86: 5768–5772 (1989).
Rouault TA, Teng CK, Kaptain S, Burgess WH, Haile DJ, Samaniego F, Mc Bride OW, Harford JB, Klausner RD: Cloning of the cDNA encoding an RNA regulatory protein — the human iron-responsive element-binding protein. Proc Natl Acad Sci USA 87: 7958–7962 (1990).
Sczekan SR, Joshi JG: Isolation and characterization of ferritin from soyabeans. J Biol Chem 262: 13780–13788 (1987).
Seckbach S: Studies on the deposition of plant ferritin as influenced by supply of iron to iron-deficient beans. J Ultrastructure Res 22: 313–423 (1968).
Seckbach S. Ferreting out the secrets of plant ferritin — A review. J Plant Nutr 5: 369–394 (1982).
Shull GE, Theil EC: Translational control of ferritin synthesis by iron in embryonic reticulocytes of the bullforg. J Biol Chem 257: 14187–14191 (1982).
Spence MJ, Henzl MT, Lammers PJ: The structure of a Phaseolus vulgaris cDNA encoding the iron storage ferritin. Plant Mol Biol 117: 499–504 (1991).
Theil EC: Ferritin: structure, gene regulation, and cellular function in animals, plants and microorganisms. Annu Rev Biochem 56: 289–315 (1987).
Theil EC: Regulation of ferritin and transferrin receptor mRNAs. J Biol Chem 265: 4771–4774 (1990).
van derMark F, deLange T, Bienfait HF: The role of ferritin in developing primary bean leaves under various light conditions. Planta 153: 338–342 (1981).
van derMark F, Bienfait HF, van derEnde H: Variable amounts of translatable ferritin mRNA in bean leaves with various iron contents. Biochem Biophys Res Commun 115: 463–469 (1983).
van derMark F, van derBriel W, Huisman HG: Phytoferritin is synthesized in vitro as a high-molecular-weight precursor. Biochem J 214: 943–950 (1983).
Walden WE, Thach RE: Translational control of gene expression in a normal fibroplast. Characterization of a subclass of mRNAs with unusual kinetic properties. Biochemistry 25: 2033–2041 (1986).
Walden WE, Patino MM, Gaffield L: Purification of a specific repressor of ferritin mRNA translation from rabbit liver. J Biol Chem 264: 13765–13769 (1989).
Wang YH, Sczekan SR, Theil EC. Structure of the 5′ untranslated regulatory region of ferritin mRNA studied in solution. Nucl Acids Res 18: 4463–4468 (1990).
Westhoff P, Nelson N, Bünemann H, Herrmann RG: Localization of genes for coupling factor subunits on the spinach plastid chromosome. Curr Genet 4: 109–120 (1981).
White K, Munro HN: Induction of ferritin subunit synthesis by iron is regulated at both the transcriptional and translational levels. J Biol Chem 263: 8938–8942 (1988).
Zahringer J, Baliga BS, Munro HN: Novel mechanism for translational control in regulation of ferritin synthesis by iron. Proc Natl Acad Sci USA 73: 857–861 (1976).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lobreaux, S., Massenet, O. & Briat, JF. Iron induces ferritin synthesis in maize plantlets. Plant Mol Biol 19, 563–575 (1992). https://doi.org/10.1007/BF00026783
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00026783