Summary
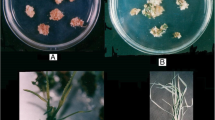
The genetic control of plant regeneration from callus culture was studied in tetraploid alfalfa (Medicago sativa L.). Seven cultivars (total 72 plants) were screened for regenerability. Ladak had the best regeneration response, in which 42% of the plants regenerated. Four regenerable plants and three nonregenerable plants were used to form 10F1 hybrids and three S1 populations. Segregation ratios in the populations suggested that regenerability of alfalfa via petiole culture was under the control of two complementary genes, Rn3 and Rn4. The presence of both dominant genes was necessary for a plant to regenerate in a two-step culture system. The data also indicated that gene dosage influenced regeneration efficiency. Significant reciprocal effects demonstrated that the interaction between callus induction medium and callus regenerability was affected by cytoplasmic factor(s).
Similar content being viewed by others
References
Atanassov, A. & D.C.W. Brown, 1984. Plant regeneration from suspension culture and mesophyll protoplasts of Medicago sativa L. Plant Cell Tissue Organ Culture 3: 149–162.
Bingham, E.T., L.V. Hurley, D.W. Kaatz & J.W. Saunders, 1975. Breeding alfalfa which regenerates from callus tissue in culture. Crop Sci 15: 719–721.
Brown, D.C.W. & A. Atanassov, 1985. Role of genetic background in somatic embryogenesis in Medicago. Plant Cell Tissue Organ Culture 4: 111–122.
Buiatti, M., S. Baronocelli, A. Bennici, M. Pagliai & R. Tesi, 1974. Genetics of growth and differentiation in vitro of Brassica oleracea var. botrytis. II. An in vitro and in vivo analysis of a diallel cross. Z Pflanzenzüchtg 72: 269–274.
Campbell, C.T. & D.A. Tomes, 1984. Establishment and multiplication of red clover plants by in vitro shoot tip culture. Plant Cell Tissue Organ Culture 3: 49–57.
Hlasnikova, A., 1977. Androgenesis in vitro evaluated from the aspects of genetics. Z Pflanzenzüchtg 78: 44–56.
Izhar, S. & J.B. Power, 1977. Genetical studies with petunia leaf protoplasts. I. Genetic variation to specific growth hormones and possible genetic control on stages of protoplast development in culture. Plant Sci Lett 8: 375–383.
Kao, K.N. & M.R. Michayluk, 1981. Embryoid formation in alfalfa cell suspension cultures from different plants. In Vitro 17: 645–648.
Keyes, G.T. & E.T. Bingham, 1979. Heterosis and ploidy effects on the growth of alfalfa callus. Crop Sci 19: 473–476.
Kumar, A.S., T.P. Reddy & G.M. Reddy, 1983. Plantlet regeneration from different callus cultures of pigeon pea (Cajanus cajan L.). Plant Sci Lett 32: 271–278.
Liang, G.H., N. Sangduen, E.G. Heyne & R.G. Sears, 1982. Polyhaploid production through anther culture in common wheat. J Heredity 73: 360–364.
Ma, H., M. Gu and G.H. Liang, 1987. Plant regeneration from cultured immature embryos of Sorghum bicolor (L.) Moench. Theor Appl Genet 73: 389–394.
Mitten, D.H., S.J. Sato & T.A. Skokut, 1984. In vitro regeneration potential of alfalfa germplasm sources. Crop Sci 24: 943–945.
Murashige, T., 1974. Plant propagation through tissue cultures. Ann Rev Plant Physiol 25: 135–166.
Nesticky, M., F.J. Novak, A. Piovarci & M. Dolezelova, 1983. Genetic analysis of callus growth of maize (Zea mays L.) in vitro. Z Pflanzenzüchtg 91: 322–328.
Oelck, M.M. & O. Schieder, 1983. Genotypic differences in some legume species affecting the redifferentiation ability from callus to plants. Z Pflanzenzüchtg 91: 312–321.
Phillips, G.C., 1983. Screening alfalfas adapted to the southwestern United States for regenerator genotypes. In Vitro 19: 265.
Reisch, B. & E.T. Bingham, 1980. The genetic control of bud formation from callus cultures of diploid alfalfa. Plant Sci Letters 20: 71–77.
Saunders, J.W. & E.T. Bingham, 1975. Growth regulator effects on bud initiation in callus cultures of Medicago sativa. Am J Bot 62: 850–855.
Schenk, R.V. & A.C. Hildebrandt, 1972. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can J Bot 50: 199–204.
Sun, M.H. & A.J. Ullstrup, 1971. In vitro growth of corn endosperm. Bull of Torrey Bot Club 98: 251–258.
Tabata, M. & F. Motoyoshi, 1965. Hereditary control of callus formation in maize endosperm cultured in vitro. Jap J Genet 40: 343–355.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wan, Y., Sorensen, E.L. & Liang, G.H. Genetic control of in vitro regeneration in alfalfa (Medicago sativa L.). Euphytica 39, 3–9 (1988). https://doi.org/10.1007/BF00025103
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00025103