Abstract
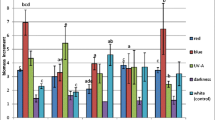
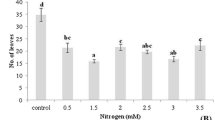
Nicotiana plumbaginifolia suspension cultured cells were grown on medium supplemented with valine, leucine and isoleucine, singly or in combination. The effects of the three branched-chain amino acids on cell growth rate and on the activity of acetohydroxyacid synthase (AHAS), the first enzyme (and the main regulative site) of their biosynthetic pathway, were studied. Results showed that valine and leucine, at concentrations ranging from 10−4 to 10−3 M, inhibit growth, and at higher doses (from 10−2 to 10−1 M) AHAS activity. Growth, but not AHAS activity, was affected also by isoleucine. The addition of ammonium succinate to the culture medium, in order to counteract a possible general inhibitory effect of these compounds on nitrogen metabolism, relieved only partially their cytotoxicity. Feeding cells with equimolar mixtures of the three amino acids resulted in a minor but reproducible decrease in AHAS level, which was proportional to the dose. A similar result was obtained also on N. plumbaginifolia seedlings, suggesting that in this species a modulation of enzyme level could play a role in controlling the flow of metabolites through the pathway.
Similar content being viewed by others
Abbreviations
- AHAS:
-
acetohydroxyacid synthase
- BCAA:
-
branched-chain amino acids
- FAD:
-
flavin adenine dinucleotide
- GS:
-
glutamine synthetase
- TPP:
-
thiamine pyrophosphate
References
Barak Z, Kogan N and Gollop N and Chipman DM (1990) Importance of AHAS isozymes in branched chain amino acid biosynthesis. In: Barak Z, Chipman DM, Schloss JV (eds) Biosynthesis of branched chain amino acids, pp91–107, VCH, Weinheim
Behrend J, Mateles R (1975) Nitrogen metabolism in plant cell suspension culture. I. Effect of amino acids on growth. Plant Physiol 56: 584–589
Bonner CA, Rodrigues AM, Miller JA, Jensen RA (1992) Amino acids are generally growth inhibitors of Nicotiana silvestris in tissue culture. Physiol Plant 84: 319–328
Borstlap AC (1981) Interaction between the branched-chain amino acids in the growth of Spirodela polyrhiza. Planta 151: 314–319
Bourgin JP (1976) Valine-induced inhibition of growth of haploid tobacco protoplasts and its reversal by isoleucine. Z Naturforsch 31: 337–338
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254
Bryan JK (1980) Synthesis of the aspartate family and branched-chain amino acids. In: Miflin BJ (ed) The biochemistry of plants: a comprehensive treatise, Vol. V, Amino acids and derivatives, pp403–452. Academic Press, New York
De Felice M, Lago CT, Squires CH, Calvo JM (1982) Acetohydroxy acid synthase isoenzymes of Escherichia coli K12 and Salmonella typhimurium. Ann Microbiol (Paris) 133A: 251–256
Dougall K (1970) Threonine deaminase from Paul's scarlet rose tissue cultures. Phytochemistry 9: 959–964
Durner J, Böger P (1990) Oligomeric forms of plant acetolactate synthase depend on flavin adenine dinucleotide. Plant Physiol 93: 1027–1031
Eoyang L, Silverman PM (1984) Purification and subunit composition of acetohydroxyacid synthase I from Escherichia coli K12. J Bacteriol 157: 184–189
Forlani G, Riccardi G, De Rossi E, De Felice M (1991) Biochemical evidence for multiple forms of acetohydroxyacid synthase in Spirulina platensis. Arch Microbiol 155: 298–302
Gamborg OL, Millar RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50: 151–158
Hayzer DJ, Moses V (1978) The enzymes of proline biosynthesis in Escherichia coli. Biochem J 173: 219–228
Heimer YM, Filner P (1970) Regulation of the nitrate assimilation pathway of cultured tobacco cells. Biochim Biophys Acta 215: 152–165
Holmberg S, Petersen JGL (1988) Regulation of isoleucinevaline biosynthesis in Saccharomyces cerevisiae. Curr Genet 13: 207–218
Miflin BJ (1969) A technique for the sterile culture of germinating barley embryos. J Exp Bot 20: 805–809
Miflin BJ (1969) The inhibitory effects of various amino acids on the growth of barley seedlings. J Exp Bot 20: 810–819
Miflin BJ (1971) Cooperative feedback control of barley acetohydroxyacid synthetase by leucine, isoleucine and valine. Arch Biochem Biophys 146: 542–550
Miflin BJ, Cave PR (1972) The control of leucine, isoleucine and valine biosynthesis in a range of higher plants. J Exp Bot 23: 511–516
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio-assay with tobacco tissue cultures. Physiol Plant 15: 473–497
Nielsen E, Forlani G, Cella R, Parisi B (1986) Biochemical characterization of the natural resistance of rice to the proline analogue azetidin-2-carboxylic acid. Plant Sci 44: 147–154
Nielsen E, Rollo F, Parisi B, Cella R, Sala F (1979) Genetic markers in cultured plant cells: differential sensitivities to amethopterin, azetidin-2-carboxylic acid and hydroxyurea. Plant Sci Lett 15: 113–125
Pinto JEBP, Dyer WE, Weller SC, Herrmann KM (1988) Glyphosate induces 3-deoxy-D-arabino-heptulosonate-7-phosphate synthase in potato (Solanum tuberosum L.) cells grown in suspension culture. Plant Physiol 87: 891–893
Rognes SE, Wallsgrove RM, Kueh JSH, Bright SWJ (1986) Effects of exogenous amino acids on growth and activity of four aspartate pathway enzymes in barley. Plant Sci 43: 45–50
Sakano K (1979) Derepression and repression of lysinesensitive aspartokinase during in vitro culture of carrot root tissue. Plant Physiol 63: 583–585
Squires CT, Levinthal M, De Felice M (1981) A role for threonine deaminase in the regulation of α-acetolactate biosynthesis in Escherichia coli K12. J Gen Microbiol 127: 19–26
Thompson GA, Datko AH, Mudd SH, Giovanelli J (1982) Methionine biosynthesis in Lemna. Plant Physiol 69: 1077–1083
Umbarger HE (1983) The biosynthesis of isoleucine and valine and its regulation. In: Herrmann KM and Somerville RL (eds) Amino acids: biosynthesis and genetic regulation, pp245–266. Addison-Wesley, Reading, MA
Umbarger HE (1987) Biosynthesis of the branched-chain amino acids. In: Neidhardt FC, Ingraham JL, Low BL, Magasanick B, Schaechter M and Umbarger HE (eds) Escherichia coli and Salmonella typhimurium. Cellular and molecular biology, Vol. I, pp352–367. American Society for Microbiology, Washington
Wallsgrove RM (1990) The biochemistry and genetics of branched chain amino acid biosynthesis in higher plants. In: Barak Z, Chipman DM and Schloss JV (eds) Biosynthesis of branched chain amino acids, pp43–51. VCH, Weinheim
Wallsgrove RM, Risiott R, King J, Bright SJW (1986) Biochemical characterisation of Nicotiana plumbaginifolia auxotrophs that require branched-chain amino acids. Plant Cell Rep 3: 223–226
Westerfeld WW (1945) A colorimetric determination of blood acetoin. J Biol Chem 161: 495–502
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Forlani, G., Suardi, M.C., Parisi, B. et al. Regulatory effects of exogenous branched-chain amino acids in Nicotiana plumbaginifolia cell suspension cultures. Plant Growth Regul 14, 203–209 (1994). https://doi.org/10.1007/BF00024794
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00024794