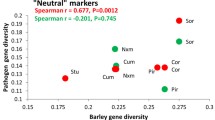
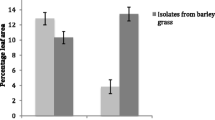
Summary
Coevolution refers to reciprocal genetic changes that occur in two or more ecologically interacting species. In agricultural ecosystems, we are especially concerned with the genetic response of pathogen populations to resistant cultivars produced by plant breeding programs. It would be useful to be able to predict whether disease resistance is likely to be durable or ephemeral before a cultivar is widely grown. Though it may not be possible to predict durability in advance, knowledge of the genetic structure of pathogen populations may prove useful for making predictions about the rate at which pathogens adapt to resistant varieties. Much has been learned about the genetic structure of populations of obligate fungal pathogens such as rusts and mildews, which have become paradigms for plant pathology. We have focused our effort on the population genetics of the less known, non-specialized, necrotrophic pathogens, such as the Septorias of small grains. Our approach has been to use DNA fingerprinting and RFLP analysis to conduct field experiments that elucidate how populations of fungal pathogens adapt in agroecosystems. Our results suggest that mating system may have a greater impact than natural selection on the genetic structure of populations of Mycosphaerella graminicola (anamorph Septoria tritici).
Similar content being viewed by others
References
Ahmed H.U., C.C. Mundt & S.M. Coakley, 1995. Host-pathogen relationship of geographically diverse isolates of Septoria tritici and wheat cultivars. Plant Pathol 44: 838–847.
Ahmed H.U., C.C. Mundt, M.E. Hoffer & S.M. Coakley, 1996. Selective influence of wheat cultivars on the pathogenicity of Mycosphaerella graminicola (anamorph Septoria tritici). Phytopathology 86: 454–458.
Boeger J.M., R.S. Chen & B.A. McDonald, 1993. Gene flow between geographic populations of Mycossphaerella graminicola (anamorph Septoria tritici) detected wit RFLP markers. Phytopathology 83: 1148–1154.
Brokenshire T., 1975. The role of graminaceous species in the epidemiology of Septoria tritici on wheat. Plant Pathol 24: 33–38.
Brown J.K.M. & M.S. Wolfe, 1990. Structure and evolution of a population of Erisyphe graminis f.sp. hordei. Plant Pathol 39: 376–390.
Chen R.S. & B.A. McDonald, 1996. Sexual reproduction plays a major role in the genetic structure of populations of the fungus Mycosphaerella graminicola. Genetics 142: 1119–1127.
Chen R.S., J.M. Boeger & B.A. McDonald, 1994. Genetic stability in a population of a plant pathogenic fungus over time. Mol Ecol 3: 209–218.
Chin K.M. & M.S. Wolfe, 1984. Selection on Erisyphe graminis in pure and mixed stands of barley. Plant Pathol 33: 535–546.
Engels, B., 1988. FISH6. Version 1.001. University of Wisconsin Genetics Department, Madison, WI 53706.
Eyal Z., A.L. Scharen, M.D. Huffman & J.M. Prescott, 1985. Global insights into virulence frequencies of Mycosphaerella graminicola. Phytopathology 75: 1456–1462.
Flor H.H., 1956. The complementary genic systems in flax and flax rust. Advanc Genet 8: 29–54.
Futuyma D.J. & M.S. Slatkin, 1983. Coevolution. Sinauer Associates, Sunderland, MA.
Goodwin S.B., L.S. Sujkowski & W.E. Fry, 1995. Rapid evolution of pathogenicity within clonal lineages of the potato late blight disease fungus. Phytopathology 85: 669–676.
Groth J.V. & E.A. Ozmon, 1994. Contrasting effects of asexual reproduction and random mating on changes in virulence frequency in a field collection of Uromyces appendiculates. Phytopathology 84: 566–569.
King J.E., R.J. Cook & S.C. Melville, 1983. A review of Septoria diseases of wheat and barley. Ann Appl Biol 103: 345–373.
Kolmer J.A., 1993. Selection in a heterogeneous population of Puccinia recondita f.sp. tritici. Phytopathology 83: 909–914.
Kolmer J.A., J.Q. Liu & M. Sies, 1995. Virulence and molecular polymorphism in Puccinia recondita f.sp. tritici in Canada. Phytopathology 85: 276–285.
McDermott J.M. & B.A. McDonald, 1993. Gene flow in plant pathosystems. Ann Rev Phytopathol 31: 353–373.
McDonald B.A. & J.P. Martinez, 1990a. DNA restriction fragment length polymorphisms among Mycosphaerella graminicola (anamorph Septoria tritici) isolates collected from a single wheat field. Phytopathology 80: 1368–1373.
McDonald B.A. & J.P. Martinez, 1990b. Restriction fragment length polymorphisms in Septoria tritici occur at a high frequency. Curr Gen 17: 133–138.
McDonald B.A. & J.P. Martinez, 1991a. Chromosome length polymorphisms in a Septoria tritici population. Curr Gen 19: 265–271.
McDonald B.A. & J.P. Martinez, 1991b. DNA fingerprinting of the plant pathogenic fungus Mycosphaerella graminicola (anamorph Septoria tritici). Exp Mycol 15: 146–158.
McDonald B.A., R.E. Pettway, R.S. Chen, J.M. Boeger & J.P. Martinez, 1995. The population genetics of Septoria tritici (teleomorph Mycosphaerella graminicola). Can J Bot 73 (supplement): S292-S301.
Schuh W., 1990. Influence of tillage systems on disease intensity and spatial pattern of Septoria leaf blotch. Phytopathology 80: 1337–1340.
Shaw M.W. & D.J. Royle, 1989. Airborne inoculum as a major source of Septoria tritici (Mycosphaerella graminicola) infections in winter wheat crops in the UK. Plant Pathol 38: 35–43.
Stakman E.C. & J.J. Christensen, 1960. The problem of breeding resistant varieties. Plant Pathology: An Advanced Treatise 3: 567–624.
Staskawicz B.J., F.M. Ausubel, B.J. Baker, J.G. Ellis & J.D.G. Jones, 1995. Molecular genetics of plant disease resistance. Science 268: 661–667.
Stoddart J.A. & J.F. Taylor, 1988. Genotypic diversity: estimation and prediction in samples. Genetics 118: 705–711.
Weir B.S., 1990. Genetic Data Analysis. Sinauer Associates, Sunderland, MA.
Zeigler R.S., L.X. Cuoc, R.P. Scott, M.A. Bernardo, D.H. Chen, B. Valent & R.J. Nelson, 1995. The relationship between lineage and virulence in Pyricularia grisea in the Philippines. Phytopathology 85: 443–451.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McDonald, B.A., Mundt, C.C. & Chen, RS. The role of selection on the genetic structure of pathogen populations: Evidence from field experiments with Mycosphaerella graminicola on wheat. Euphytica 92, 73–80 (1996). https://doi.org/10.1007/BF00022831
Issue Date:
DOI: https://doi.org/10.1007/BF00022831