Abstract
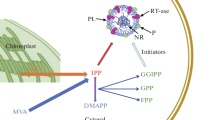
Commercially used natural rubber (cis-1,4-polyisoprene) is a secondary metabolite of the rubber tree (Hevea brasiliensis). Previous studies have shown the involvement of a prenyl transferase in the final steps of natural rubber biosynthesis which includes polymerization of isopentenyl pyrophosphate into rubber. Using synthetic oligonucleotides corresponding to the partial amino acid sequences of this protein as probes to screen a laticifer-specific cDNA library, we have isolated a full-length cDNA which encodes a 47 kDa protein with strong homology to farnesyl diphosphate synthases from many species. The catalytic activity of this protein was confirmed by complementing the deletion yeast mutant. In Hevea, this gene is expressed in latex producing cells and in the epidermal region of the rubber plant suggesting a dual role for the protein in the biosyntheses of rubber and other isoprenoids. Although the expression level of this gene is not significantly affected by hormone treatment (e.g. ethylene), regeneration of latex due to tapping increases its expression level.
Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ: Basic local alignment search tool. J Mol Biol 215: 403–410 (1990).
Anderson MS, Yarger JG, Burck CL, Poulter CD: Farnesyl diphosphate synthetase: molecular cloning, sequence, and expression of an essential gene from Saccharomyces cerevisiae. J Biol Chem 264: 19176–19184 (1989).
Archer BL, Audley BG: Some new aspects of rubber biosynthesis. Bot J Linnean Soc 94: 181–196 (1987).
Archer BL, Audley BG: Biosynthesis of rubber. Adv Enzymol 29: 221–257 (1967).
Archer BL, Cockbain EG: Rubber transferase from Hevea brasiliensis latex. Meth Enzymol 15: 476–480 (1969).
Ashby MN, Edwards PA: Identification and regulation of a rat liver cDNA encoding farnesyl pyrophosphate synthetase. J Biol Chem 264: 635–640 (1989).
Attuci S, Aitken SM, Ibrahim RK, Gulick PJ: A cDNA encoding farnesyl pyrophosphate synthase in white lupin. Plant Physiol 108: 835–836 (1995).
Bernatzky R, Tanksley SD: Genetics of actin-related sequences in tomato. Theor Appl Genet 72: 314–321 (1986).
Boutry M, Chua NH: A nuclear gene encoding the beta subunit of the mitochondrial ATPase synthase in Nicotiana plumbaginifolia. EMBO J 4: 2159–2165 (1985).
Broekaert W, Lee HI, Kush A, Chua NH, Raikhel N: Wound-induced accumulation of mRNA containing a hevein sequence in laticifers of rubber tree (Hevea brasiliensis). Proc Natl Acad Sci USA 87: 7633–7637 (1990).
Chye ML, Kush A, Tan CT, Chua NH: Characterization of cDNA and genomic clones encoding 3-hydroxy-3-methylglutaryl-coenzyme A reductase from Hevea brasiliensis. Plant Mol Biol 16: 567–577 (1991).
Chye ML, Tan CT, Chua NH: Three genes encode 3-hydroxy-3-methylglutaryl-coenzyme A reductase in Hevea brasiliensis: hmg1 and hmg3 are differentially expressed. Plant Mol Biol 19: 473–484 (1992).
Clarke CF, Tanaka RD, Svenson K, Wamsley M, Fogelman AM, Edwards PA: Molecular cloning and sequence of a cholesterol repressible enzyme related to prenyl transferase in the isoprene biosynthetic pathway. Mol Cell Biol 7: 3138–3146 (1987).
Cornish K: The separate roles of plant cis and trans prenyl transferases in cis-1–4 polyisoprene biosynthesis. Eur J Biochem 218: 267–271 (1993).
Coupe M, Chrestin H: The hormonal stimulation of latex yield: physico-chemical and biochemical mechanisms of hormonal (ethylene) stimulation. In: d'Auzac J, Jacob JL, Chrestin H (eds) Physiology of Rubber Tree Latex, pp 295–319. CRC Press, Boca Raton, FL (1989).
Fay Ede, Jacob JL: Anatomical organization of the laticiferous system in bark. In: d'Auzac J, Jacob JL, Chrestin H (eds) Physiology of Rubber Tree Latex, pp. 3–14. CRC Press, Boca Raton, FL (1989).
Delourme D, Lacroute F, Karst F: Cloning of an Arabidopsis thaliana cDNA coding for farnesyl diphosphate synthase by functional complementation in yeast. Plant Mol Biol 26: 1867–1873 (1994).
Dennis MS and Light DR: Rubber elongation factor from Hevea brasiliensis: identification, characterization, and role in rubber biosynthesis. J Biol Chem 264: 18608–18617 (1989).
Feinberg AP, Vogelstein B: A technique for radiolabelling restriction endonuclease fragments to high specific activity. Anal Biochem 132: 6–13 (1983).
Funkhouser JM: Immunohistochemical analysis of transgenic tissues. In: Murphy D, Carter DA (eds) Methods in Molecular Biology. Transgenesis Techniques Principles and Protocols, pp. 395–406. Humana Press (1993).
Gish W, States DJ: Identification of protein coding regions by database similarity search. Nature Genet 3: 266–272 (1993).
Goyvaerts E, Dennis M, Light D, Chua N-H: Cloning and sequencing of the cDNA encoding the rubber elongation factor of Hevea brasiliensis. Plant Physiol: 317–321 (1991).
Jacob JL: Particularité de la glycolyse et de sa régulation au sein du latex d'Hevea brasiliensis. Physiol Veg 8: 395–411 (1970).
Jacob JL, Prevot JC, Roussel R, Lacrotte R, Serres E, d'Auzac J, Eschbach JM, Omont H: Yield limiting factor, latex physiological parameters, latex diagnosis, and clonal typology. In: d'Auzac J, Jacob JL, Chrestin H (eds) Physiology of Rubber Tree Latex, pp. 345–382. CRC Press, Boca Raton, FL (1989).
Jennings SM, Tsay YH, Fisch TM, Robinson GW: Molecular cloning and characterization of the yeast gene for squalene synthetase. Proc Natl Acad Sci USA 88: 6038–6042 (1991).
John P: Rubber. In: Biosynthesis of the Major Crop Products, pp. 114–126, John Wiley, Chichester, New York (1992).
Kleinig H: The role of plastids in isoprenoid biosynthesis. Annu Rev Plant Mol Biol 40: 39–59 (1989).
Kuntz M, Romer S, Suire C, Hugueney P, Weil J, Schantz R, Camara B: Identification of a cDNA for the plastid-located geranylgeranyl pyrophosphate synthase from Capsicum annuum: correlative increase in enzyme activity and transcript level during fruit ripening. Plant J 2: 25–34 (1992).
Kush A: Isoprenoid biosynthesis: the Hevea factory. Plant Physiol Biochem 32: 761–767 (1994).
Kush A, Goyvaerts E, Chye ML, Chua NH: Laticifer-specific gene expression in Hevea brasiliensis (rubber tree). Proc Natl Acad Sci USA 87: 1787–1790 (1990).
Light DR, Dennis MS: Purification of a prenyltransferase that elongates cis-polyisoprene rubber from the latex of Hevea brasiliensis. J Biol Chem 264: 18589–18597 (1989).
Light DR, Lazarus RA, Dennis MS: Rubber elongation by farnesyl pyrophosphate synthases involves a novel switch in enzyme stereospecificity. J Biol Chem 264: 18598–18607 (1989).
Nivison HI, Hanson MR: Production and purification of synthetic peptide antibodies. Plant Mol Biol Rep 5: 295–309 (1987).
Pujade-Renaud V, Clement A, Perrot-Rechenmann C, Prevot J-C, Chrestin H, Jacob J-L, Guern J: Ethylene-induced increase in glutamine synthetase activity and mRNA levels in Hevea brasiliensis latex cells. Plant Physiol 105: 127–132 (1994).
Ross AB, Broach JR: Propagation and expression of cloned gene in yeast: 2μm circle based vectors. Meth Enzymol 185: 234–279 (1967).
Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY (1989).
Stermar BA, Bianchini GM, Korth KL: Regulation of HMG-CoA reductase activity in plants. J Lipid Res 35: 1133–1140 (1994).
Stryer L: Biosynthesis of membrane lipids and steroid hormones. In: Biochemistry 2nd ed, pp. 457–483. WH Freeman, New York (1975).
Venkatachalam KV, Greenblatt GA, Benedict CR: Enzymatic synthesis of a rubber polymer. In: Petroski RJ, McCormic SP (eds) Secondary Metabolite Biosynthesis and Metabolism, pp. 351–359. Plenum Press, New York (1992).
Wilkin DJ, Kutsunai SY, Edwards PA: Isolation and sequence of the human farnesyl pyrophosphate synthetase cDNA coordinate regulation of the mRNAs for farnesyl pyrophosphate synthetase, 3-hydroxy-3-methylglutaryl coenzyme A reductase, and 3-hydroxy-3-methylglutaryl coenzyme A synthase by phorbol ester. J Biol Chem 265: 4607–4614 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Adiwilaga, K., Kush, A. Cloning and characterization of cDNA encoding farnesyl diphosphate synthase from rubber tree (Hevea brasiliensis). Plant Mol Biol 30, 935–946 (1996). https://doi.org/10.1007/BF00020805
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00020805