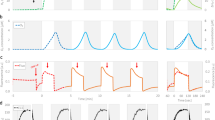
Abstract
Photosystem I-driven cyclic electron transport was measured in intact cells of Synechococcus sp PCC 7942 grown under different light intensities using photoacoustic and spectroscopic methods. The light-saturated capacity for PS I cyclic electron transport increased relative to chlorophyll concentration, PS I concentration, and linear electron transport capacity as growth light intensity was raised. In cells grown under moderate to high light intensity, PS I cyclic electron transport was nearly insensitive to methyl viologen, indicating that the cyclic electron supply to PS I derived almost exclusively from a thylakoid dehydrogenase. In cells grown under low light intensity, PS I cyclic electron transport was partially inhibited by methyl viologen, indicating that part of the cyclic electron supply to PS I derived directly from ferredoxin. It is proposed that the increased PSI cyclic electron transport observed in cells grown under high light intensity is a response to chronic photoinhibition.
Similar content being viewed by others
Abbreviations
- DBMIB:
-
2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone
- DCMU:
-
3-(3,4-dichlorophenyl)-1,1-dimethylurea
- ES:
-
energy storage
- MV:
-
methyl viologen
- PAm :
-
photoacoustic thermal signal with strong non-modulated background light added
- PAs :
-
photoacoustic thermal signal without background light added
References
Arnon DI (1959) Conversion of light into chemical energy in photosynthesis. Nature 184: 10–21
Arnon DI (1991) Photosynthetic electron transport: Emergence of a concept, 1949–59. Photosynth Res 29: 117–131
Canaani O (1990) The role of cyclic electron flow around Photosystem I and excitation energy distribution between the photosystems upon acclimation to high ionic stress in Dunaliella salina. Photochem Photobiol 52: 591–599.
Canaani O, Schuster G and Ohad I (1989) Photoinhibition in Chlamydomonas reinhardtii: Effect on state transition, intersystem energy distribution, and Photosystem I cyclic electron flow. Photosynth Res 20: 129–146
Cha Y and Mauzerall DC (1992) Energy storage of linear and cyclic electron flows in photosynthesis. Plant Physiol 100: 1869–1877
Collier JL, Herbert SK, Fork DC and Grossman AR (1994) Changes in the cyanobacterial photosynthetic apparatus during acclimation to macronutrient deprivation. Photosynth Res 42: 173–183
Fork DC and Herbert SK (1991) A gas-permeable photoacoustic cell. Photosynth Res 27: 151–157
Fork DC and SKHerbert (1993a) Mini Review: Electron transport and photophosphorylation by Photosystem I in vivo in plants and cyanobacteria. Photosynth Res 36: 149–168
Fork DC and SKHerbert (1993b) Yearly review: The application of photoacoustic techniques to studies of photosynthesis. Photochem Photobiol 57: 207–220
Fork DC, Bose S and Herbert SK (1986) Radiationless transitions as a protection mechanism against photoinhibition in higher plants and a red alga. Photosynth Res 10: 327–333
Furbank RT, Jenkins CLD and Hatch MD (1990) C4 photosynthesis: Quantum requirement, C4 acid overcycling and Q-cycle involvement. Aust J Plant Physiol 17: 1–7
Golbeck JH and Bryant DA (1991) Photosystem I. Curr Top Bioenerg 16: 83–177
Gu T-Q, Iwama Y, Murakami A, Adhikary SP and Fujita Y (1994) Changes in the cytochrome c oxidase activity in response to light regime for photosynthesis observed with the cyanophyte Synechocystis PCC 6714. Plant Cell Physiol 35: 1135–1140
Harbinson J and Foyer CH (1991) Relationships between the efficiencies of Photosystems I and II and stromal redox state in CO2-free air. Plant Physiol 97: 41–49
Heber U and Walker D (1992) Concerning a dual function of coupled cyclic electron transport in leaves. Plant Physiol 100: 1621–1626
Herbert SK, Fork DC and Malkin S (1990) Photoacoustic measurements in vivo of energy storage by cyclic electron flow in algae and higher plants. Plant Physiol 94: 926–934
Herbert SK, Samson G, Fork DC and Laudenbach DE (1992) Characterization of damage to Photosystems I and II in a cyanobacterium lacking detectable iron superoxide dismutase activity. Proc Natl Acad Sci USA 89: 8716–8720
Hind G, Shahak Y and Slovacek RE (1981) The function and mechanism of cyclic electron transport. In: Akoyunoglou G (ed) Photosynthesis II. Electron Transport and Photophosphorylation, pp 97–97. Balaban International Science Services, Philadelphia
Jeanjean R, Mathijs HCP, Onana B, Havaux M and Joset F (1993) Exposure of the cyanobacterium Synechocystis PCC 6803 to salt stress induces concerted changes in respiration and photosynthesis. Plant Cell Physiol 34: 1073–1079
Katona E, Neimanis S, Schönknecht G and Heber U (1992) Photosystem I-dependent cyclic electron transport is important in controlling Photosystem II activity in leaves under conditions of water stress. Photosynth Res 34: 449–464
Klughammer C and Schreiber U (1991) Analysis of light-induced absorbance in the near-infrared spectral region. I. Characterization of various components in isolated chloroplasts. Z Naturforsch 46c: 233–244
Krause GH and Behrend U (1986) ΔpH-dependent chlorophyll fluorescence quenching indicating a mechanism of protection against photoinhibition of chloroplasts. FEBS Lett 200: 298–302
Krause GH and Wies E (1991) Chlorophyll fluorescence and photosynthesis: The basics. Ann Rev Plant Physiol Plant Molec Biol 42: 313–349
Laudenbach DE, Herbert SK, McDowell C, Fork DC, Grossman AR and Strauss NA (1990) Cytochrome c553 is not required for photosynthetic activity in the cyanobacterium Synechococcus. Plant Cell 2: 913–924
Malkin S, Lasser-Ross N, Bults G and Cahen D (1981) Photoacoustic spectroscopy in photosynthesis. In: Akoyunoglou G (ed) Photosynthesis III. Structure and Molecular Organization of the Photosynthetic Apparatus, pp 1031–1042. Balaban International Science Services, Philadelphia
Maxwell PC and Biggins J (1976) Role of cyclic electron transport in photosynthesis as measured by the turnover of P700 in vivo. Biochemistry 15: 3975–3981
Mi H, Endo T, Schreiber U and Asada K (1992a) Donation of electrons from cytosolic components to the intersystem chain in the cyanobacterium Synechococcus sp. PCC 7002 as determined by the reduction of P700+. Plant Cell Physiol 33: 1099–1105
Mi H, Endo T, Schreiber U, Ogawa T and Asada K (1992b) Electron donation from cyclic and respiratory flows to the photosynthetic intersystem chain is mediated by pyridine nucleotide dehydrogenase in the cyanobacterium Synechocystis PCC 6803. Plant Cell Physiol 33: 1233–1237
Mi H, Endo T, Schreiber U, Ogawa T and Asada K (1994) NAD(P)H dehydrogenase-dependent cyclic electron flow around Photosystem I in the cyanobacterium Synechocystis PCC 6803: A study of dark-starved cells and spheroplasts. Plant Cell Physiol 35: 163–173
Murakami A and Fujita Y (1991) Regulation of photosystem stoichiometry in the photosynthetic system of the cyanophyte Synechocystis PCC 6714 in response to light intensity. Plant Cell Physiol 32: 223–230
Myers J (1987) Is there significant cyclic electron flow around photoreaction 1 in cyanobacteria? Photosynth Res 14: 55–69
Myers J, Graham J-R and Wang RT (1980) Light harvesting in Anacystis nidulans studied in pigment mutants. Plant Physiol 66: 1144–1149
Ogawa T (1991) A gene homologous to the subunit-2 gene of NADH dehydrogenase is essential to inorganic carbon transport of Synechocystis PCC 6803. Proc Natl Acad Sci USA 88: 4274–4279
Ogawa T (1992) NAD(P)H dehydrogenase: A component of PS I cyclic electron flow driving inorganic carbon transport in cyanobacteria. In: Murata N (ed) Proceedings of the 9th International Congress on Photosynthesis, Vol III, pp 763–770. Kluwer Academic Publishers, Dordrecht, The Netherlands
Ogawa T, Miyano A and Inoue Y (1985) Photosystem-I-driven inorganic carbon transport in the cyanobacterium, Anacystis nidulans. Biochim Biophys Acta 808: 77–84
Osborne BA and Geider RJ (1988) Measurements of minimum photon requirements. Photosynth Res 16: 291–292
Oxborough K and Horton P (1988) A study of the regulation and function of energy-dependent quenching in pea chloroplasts. Biochim Biophys Acta 934: 135–143
Peltier G and Schmidt GW (1991) Chlororespiration: An adaptation to nitrogen deficiency in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 88: 4791–4795
Ravenel J, Peltier G and Havaux M (1994) The cyclic electron pathways around photosystem I in Chlamydomonas reinhardtii as determined in vivo by photoacoustic measurements of energy storage. Planta 193: 251–259
Rouag D and Dominy P (1994) State adaptations in the cyanobacterium Synechococcus 6301 (PCC): Dependence on light intensity or spectral composition? Photosynth Res 40: 107–117
Samson G, Herbert SK, Fork DC and Laudenbach DL (1994) Acclimation of the photosynthetic apparatus to growth irradiance in a mutant strain of Synechococcus lacking iron superoxide dismutase. Plant Physiol 105: 287–294
Samuelsson G, Lönneborg A, Gustafsson P and Öquist G (1987) The susceptibility of photosynthesis to photoinhibition and the capacity of recovery in high and low light grown cyanobacteria, Anacystis nidulans. Plant Physiol 83: 438–441
Topf J, Gong H, Timberg R, Mets L and Ohad I (1992) Thylakoid membrane energization and swelling in photoinhibited Chlamydomonas cells is prevented in mutants unable to perform cyclic electron flow. Photosynth Res 32: 59–69
Weis E and Berry JA (1987) Quantum efficiency of Photosystem II in relation to energy-dependent quenching of chlorophyll fluorescence. Biochim Biophys Acta 894: 198–208
Yu L, Zhao J, Mühlenhoff U, Bryant D and Golbeck JH (1993) PsaE is required for in vivo cyclic electron flow around Photosystem I in the cyanobacterium Synechococcus sp. PCC 7002. Plant Physiol 103: 171–180
Zhao J, Snyder WB, Mühlenhoff U, Rhiel E, Warren PV, Golbeck JH and Bryant DA (1993) Cloning and characterization of the psaE gene of the cyanobacterium Synechococcus sp. 7002: Characterization of a psaE mutant and overproduction of the protein in Escherichia coli. Mol Microbiol 9: 183–194
Author information
Authors and Affiliations
Additional information
CIW/DPB Publication No. 1205.
Rights and permissions
About this article
Cite this article
Herbert, S.K., Martin, R.E. & Fork, D.C. Light adaptation of cyclic electron transport through Photosystem I in the cyanobacterium Synechococcus sp. PCC 7942. Photosynth Res 46, 277–285 (1995). https://doi.org/10.1007/BF00020441
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00020441