Abstract
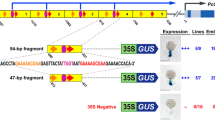
The maize Bronze2 (Bz2) gene encodes a glutathione S-transferase that is required for anthocyanin pigment accumulation. Two classes of regulatory proteins, R and C1, are required for transcriptional activation of Bz2 and several additional structural genes. Functional domains of the Bz2 promoter were identified using Bz2 promoterdriven luciferase reporter genes electroporated into maize protoplasts together with R and C1 expression plasmids. Complete regulation was conferred by 224 nt of the Bz2 promoter. Within this region at least two separable regions are independently capable of conferring regulation by R and C1. Predicted regulatory elements corresponding to two classes of sequence motifs, the Myb-box homologous ‘C1-motif’, TAACTG/CAGTTA, and the G-box and E-box homologous ‘R-motif’, CACGTG, were shown to be important for full R and C1 activation of the Bz2 promoter. Expression of reconstructed Bz2 genes with mutated promoters was quantified using RNase protection, and this analysis confirmed results obtained using reporter genes.
Similar content being viewed by others
References
Biedenkapp H, Borgmeyer U, Sippel AE, Klempnauer KH: Viral Myb oncogene encodes a sequence-specific DNA-binding activity. Nature 335: 835–837 (1988).
Bodeau JP: Molecular and genetic regulation of Bronze-2 and other maize anthocyanin genes. Ph.D. dissertation, Stanford University (1994).
Bodeau JP, Walbot V: Regulated transcription of the maize Bronze-2 promoter in electroporated protoplasts requires the C1 and R gene products. Mol Gen Genet 233: 379–387 (1992).
Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 (1976).
Callis J, Fromm M, Walbot V: Introns increase gene expression in cultured maize cells. Genes Devel 1: 1183–1200 (1987).
Carle-Urioste JC, Ko CH, Benito M, Walbot V: In vivo analysis of intron processing using splicing-dependent reporter gene assays. Plant Mol Biol 26: 1785–1795 (1994).
Chandler VL, Radicella JP, Robbins TP, Chen J, Turks D: Two regulatory genes of the maize anthocyanin pathway are homologous: isolation of B utilizing R genomic sequences. Plant Cell 1: 1175–1183 (1989).
Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159 (1987).
Christie PJ, Alfenito MR, Walbot V: Impact of low-temperature stress on general phenylpropanoid and anthocyanin pathways: enhancement of transcript abundance and anthocyanin pigmentation in maize seedlings. Planta 194: 541–549 (1994).
Coe EH, Neuffer MG, Hoisington DA: The genetics of corn. In: Sprague GF, Dudley JW (eds) Corn and Corn Improvement, 3rd ed., pp. 81–258. American Society of Agronomy, Madison, WI (1988).
Cone KC, Cocciolone SM, Burr B, Burr B: Maize anthocyanin regulatory gene pl is a duplicate of c1 that functions in the plant. Plant Cell 5: 1795–1805 (1993).
Deng WP, Nickoloff JA: Site-directed mutagenesis of virtually any plasmid by eliminating a unique site. Anal Biochem 200: 81–88 (1992).
DeVetten NC, Ferl RJ: Transcriptional regulation of environmentally inducible genes in plants by an evolutionary conserved family of G-box binding factors. Int J Biochem 26: 1055–1068 (1994).
Dooner HK, Robbins TP, Jorgensen RA: Genetic and developmental control of anthocyanin biosynthesis. Annu Rev Genet 25: 173–200 (1991).
Goff SA, Cone KC, Chandler VL: Functional analysis of the transcriptional activator encoded by the maize B gene: evidence for a direct functional interaction between two classes of regulatory proteins. Genes Devel 6: 864–875 (1992).
Grotewold E, Athma P, Peterson T: Alternatively spliced products of the maize P gene encode proteins with homology to the DNA-binding domain of myb-like transcription factors. Proc Natl Acad Sci USA 88: 4587–4591 (1991).
Grotewold E, Drummond B, Bowen B, Peterson T: The myb-homologous P gene controls phlobaphene pigmentation in maize floral organs by directly activating a flavonoid biosynthetic gene subset. Cell 76: 543–553 (1994).
Hershberger RJ, Warren CA, Walbot V: Mutator activity in maize correlates with the presence and expression of the Mu transposable element Mu9. Proc Natl Acad Sci USA 88: 10198–10202 (1991).
Jefferson RA, Burgess SM, Hirsh D: β-Glucuronidase from Escherichia coli as a gene-fusion marker. Proc Natl Acad Sci USA 83: 8447–8451 (1986).
Klein TM, Roth BA, Fromm ME: Regulation of anthocyanin biosynthetic genes introduced into intact maize tissues by microprojectiles. Proc Natl Acad Sci USA 86: 6681–6685 (1989).
Littlewood TD, Evan GI: Transcription factors 2: helix-loophelix. Protein Profile 2: 621–702 (1995).
Loake GJ, Faktor O, Lamb CJ, Dixon RA: Combination of H-box (CCTACC(N7)CT) and G-box (CACGTG) cis elements is necessary for feed-forward stimulation of a chalcone synthase promoter by the phenylpropanoid-pathway intermediate p-coumaric acid. Proc Natl Acad Sci USA 89: 9230–9234 (1992).
Ludwig SR, Habera LF, Dellaporta SL, Wessler SR: Lc, a member of the maize R gene family responsible for tissue-specific anthocyanin production, encodes a protein similar to transcriptional activators and contains the myc-homology region. Proc Natl Acad Sci USA 86: 7092–7096 (1989).
Luehrsen KR, deWet JR, Walbot V: Transient expression analysis in plants using firefly luciferase reporter gene. Meth Enzymol 216: 397–414 (1992).
Marrs KA, Alfenito MR, Lloyd AM, Walbot V: A glutathione S-transferase involved in vacuolar transfer encoded by the maize gene Bronze-2. Nature: 397–400 (1995).
Nash J, Luehrsen KR, Walbot V: Bronze-2 gene of maize: reconstruction of a wild-type allele and analysis of transcription and splicing. Plant Cell 2: 1039–1049 (1990).
Ness SA, Marknell Å, Graf T: The v-myb oncogene product binds to and activates the promyelocyte-specific mim-1 gene. Cell 59: 1115–1125 (1989).
Paz-Ares J, Ghosal D, Wienand U, Peterson PA, Saedler H: The regulatory c1 locus of Zea mays encodes a protein with homology to myb proto-oncogene products and with structural similarities to transcriptional activators. EMBO J 6: 3553–3558 (1987).
Pollak PE, Vogt T, Mo Y, Taylor LP: Chalcone synthase and flavonol accumulation in stigmas and anthers of Petunia hybrida. Plant Physiol 102: 925–932 (1993).
Roth BA, Goff SA, Klein TM, Fromm ME: C1- and R-dependent expression of the maize Bz1 gene requires sequences with homology to mammalian myb and myc binding sites. Plant Cell 3: 317–325 (1991).
Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY (1989).
Schmitz G, Theres K: Structural and functional analysis of the Bz2 locus of Zea mays: characterization of overlapping transcripts. Mol Gen Genet 233: 269–277 (1992).
Searle PF: Zinc dependent binding of a liver nuclear factor to metal response element MRE-a of the mouse metallothionein-I gene and variant sequences. Nucl Acids Res 18: 4683–4690 (1990).
Shieh MW, Wessler SR, Raikhel NV: Nuclear targeting of the maize R protein requires two nuclear localization sequences. Plant Physiol 101: 353–361 (1993).
Taylor LP, Briggs WR: Genetic regulation and photocontrol of anthocyanin accumulation in maize seedlings. Plant Cell 2: 115–127 (1990).
Tuerck JA, Fromm ME: Elements of the maize A1 promoter required for transactivation by the anthocyanin B/C1 or phlobaphene P regulatory genes. Plant Cell 6: 1655–1663 (1994).
Walbot V, Benito M, Bodeau J, Nash J: Absciscic acid induces pink pigmentation in maize aleurone tissue in the absence of Bronze-2. Maydica 39: 19–28 (1994).
Wienand U, Paz-Ares J, Scheffler B, Saedler H: Molecular analysis of gene regulation in the anthocyanin pathway of Zea mays. In: Lamb CJ, Beachy RN (eds) Plant Gene Transfer, pp. 111–124. Wiley-Liss, New York (1990).
Yu LM, Lamb CJ, Dixon RA: Purification and biochemical characterization of proteins which bind to the H-box cis-element implicated in transcriptional activation of plant defense genes. Plant J 3: 805–816 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bodeau, J.P., Walbot, V. Structure and regulation of the maize Bronze2 promoter. Plant Mol Biol 32, 599–609 (1996). https://doi.org/10.1007/BF00020201
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00020201