Abstract
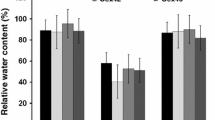
During droughting plants activate a number of genes involved in adaptation to water stress. We have isolated one such gene, btg-26, from Brassica napus. Expression of btg-26 is induced in leaf tissue within 72 h of withholding water. At 81% relative water content (RWC), when the plant is just beginning to show signs of wilting, expression is already increased six-fold over levels found in leaf tissue from fully hydrated plants. btg-26 expression reaches a maximum eleven-fold induction at 63% RWC, then transcript levels decrease as RWC continues to drop. btg-26 is also activated in plants exposed to high salinity, low temperature, heat shock and the plant hormone abscisic acid. Analysis of the deduced amino acid sequence revealed similarity to the dehydrogenase family of enzymes. These results suggest that btg-26 encodes a protein whose function may be required early during general osmotic stress in some unknown adaptive metabolic pathway.
Similar content being viewed by others
References
Allen SW, Senti-Willis AE, Maloy SR: DNA sequence of the putA gene from Salmonella typhimurium: a bifunctional membrane-associated dehydrogenase that binds DNA. Nucl Acids Res: 1676 (1993)
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ: Basic local alignment search tool. J Mol Biol 215: 403–410 (1990).
Ausubel FM, Brent R, Kinston RE, Moore DD, Seidmann IG, Smith JA, Struhl K: Current Protocols in Molecular Biology. Wiley, New York (1989).
Baker J, Steele C, DureIII L: Sequence and characterization of 6 Lea proteins and their genes from cotton. Plant Mol Biol 11: 277–291 (1988).
Biedenkapp H, Borgemeyer U, Sippel AE, Klempnauer K-H: Viral myb oncogene encodes a sequence-specific DNA-binding activity. Nature 335: 835–837 (1988).
Boyd LA, Adam L, Pelcher LE, McHughen A, Hirji R, Selvaraj G: Characterization of an Escherichia coli gene encoding betaine aldehyde dehydrogenase (BADH): structural similarity to mammalian ALDHs and plant BADH. Gene 103: 45–52 (1991).
Braun T, Bober E, Singh S, Agarwal DP, Goedde HW: Isolation and sequence analysis of a full length cDNA clone coding for human mitochondrial aldehyde dehydrogenase. Nucl Acids Res 15: 3179 (1987).
Close TJ, Kortt AA, Chandler PM: A cDNA-based comparison of dehydration-induced proteins (dehydrins) in barley and corn. Plant Mol Biol 13: 95–108 (1989).
Dellaporta SL, Wood J, Hicks JB: A plant DNA minipreparation: Version II. Plant Mol Biol Rep 1: 19–21 (1983).
Farres J, Guan KL, Weiner H: Primary structures of rat and bovine liver mitochondrial aldehyde dehydrogenases deduced from cDNA sequences. Eur J Biochem 180: 67–74 (1989).
Gasser CS, Gunning DA, Budelier KA, Brown SM: Structure and expression of cytosolic cyclophilin/peptidyl-propyl cis-trans isomerase of higher plants and production of active tomato cyclophilin in Escherichia coli. Proc Natl Acad Sci USA 87: 9519–9523 (1989).
Good AG, Maclagan JL: Effects of drought stress on the water relations in Brassica species. Can J Plant Sci 73: 525–529 (1993).
Good AG, Zaplachinski ST: The effects of drought stress on free amino acid accumulation and protein synthesis in Brassica napus. Physiol Plant 90: 9–14 (1994).
Guerrero FD, Jones JT, Mullet JE: Turgor-responsive gene transcription and RNA levels increase rapidly when pea shoots are wilted. Sequence and expression of three inducible genes. Plant Mol Biol 15: 11–26 (1990).
Guerrero FD, Mullet JE: Reduction of turgor induces rapid changes in leaf translatable RNA. Plant Physiol 88: 401–408 (1988).
Guiltinan MJ, Marcotte WR, Quatrano RS: A plant leucine zipper protein that recognizes an abscisic acid response element. Science 250: 267–271 (1990).
Hajela RK, Horvath DP, Gilmour SJ, Thomashow MF: Molecular cloning and expression of cor (cold-regulated) genes in Arabidopsis thaliana. Plant Physiol 93: 1246–1252 (1990).
Hanson AD, Hitz WD: Metabolic responses of mesophytes to plant water deficits. Annu Rev Plant Physiol 33: 163–203 (1982).
Hidalgo E, Chen YM, Lin ECC, Aguilar J: Molecular cloning and DNA sequencing of the Escherichia coli K-12 ald gene encoding aldehyde dehydrogenase. J Bact 173: 6118–6123 (1991).
Hoffman-Lieberman B, Liebermann D, Trout A, Kedes LH, Cohen SN: Human homologs of TU transposon sequences: polypurine/polypyrimidine sequence elements that can alter DNA conformation in vitro and in vivo. Mol Cell Biol 6: 3632–3642 (1986).
Holmström K-O, Welin B, Mandal A, Kristiansdottir I, Teeri TH, Lamark T, Strøm AR, Palva ET: Production of the Escherichia coli betaine-aldehyde dehydrogenase, an enzyme required for the synthesis of the osmoprotectant glycine betaine, in transgenic plants. Plant J 6: 749–758 (1994).
Imanaka T, Ohta T, Sakoda H, Widhyastuti N, Matsuoka M: Cloning, nucleotide sequence, and efficient expression of the gene coding for thermostable aldehyde dehydrogenase from Bacillus stearothermophilus, and characterization of the enzyme. J Ferment Bioeng 76: 161–167 (1993).
Jones MM, Turner NC: Osmotic adjustment in leaves of sorghum in response to water deficits. Plant Physiol 61: 122–126 (1978).
Joshi CP: An inspection of the domain between putative TATA box and translation start site in 79 plant genes. Nucl Acids Res 15: 6643–6653 (1987).
Kurkela S, Franck M: Cloning and characterization of a cold- and ABA-inducible Arabidopsis gene. Plant Mol Biol 15: 137–144 (1990).
Lam E, Chua N-H: Tetramer of a 21-base pair synthetic element confers seed expression and transcriptional enhancement in response to water stress and abscisic acid. J Biol Chem 266: 17131–17135 (1991).
Lamark T, Kaasen I, Eshoo MW, Falkenberg P, McDougall J, Strøm AR: DNA sequence and analysis of the bet genes encoding the osmoregulatory choline-glycine betaine pathway of Escherichia coli. Mol Microbiol 5: 1049–1064 (1991).
Lång V, Mäntylä E, Welin B, Sundberg B, Palva ET: Alterations in water status, endogenous abscisic acid content, and expression of rab18 gene during the development of freezing tolerance in Arabidopsis thaliana. Plant Physiol 104: 1341–1349 (1994).
Lång V, Palva ET: The expression of a rab-related gene, rab18, is induced by abscisic acid during the cold acclimation process of Arabidopsis thaliana (L.) Heynh. Plant Mol Biol 20: 951–962 (1992).
Marcotte WR, Russell SH, Quatrano RS: Abscisic acid-responsive sequences from the Em gene of wheat. Plant Cell 1: 969–976 (1989).
McKue KF, Hanson AD: Salt-inducible betaine aldehyde dehydrogenase from sugar beet: cDNA cloning and expression. Plant Mol Biol 18: 1–11 (1992).
Morgan JM: Osmoregulation and water stress in higher plants. Annu Rev Plant Physiol 35: 299–319 (1984).
Nakagoshi H, Nagase T, Kanei-Ishii C, Ueno Y, Ishii S: Binding of the c-myb proto-oncogene product to the simian virus 40 enhancer stimulates transcription. J Biol Chem 265: 3479–3483 (1990).
Niegemann E, Schulz A, Bartsch K: Molecular organization of the Escherichia coli gab cluster: nucleotide sequence of the structural genes gabD and gabP and expression of the GABA permease gene. Arch Microbiol 160: 454–460 (1993).
Nordin K, Heino P, Palva ET: Separate signal pathways regulate the expression of a low-temperature-induced gene in Arabidopsis thaliana (L.) Heynh. Plant Mol Biol 16: 1061–1071 (1991).
O'Connell MJ, Kelly JM: Physical characterization of the aldehyde-dehydrogenase-encoding gene of Aspergillus niger. Gene 84: 173–180 (1989).
Peterson JA, Lu J-Y, Geisseloder J, Graham-Lorence S, Carmona C, Witney F, Lorence MC: Cytochrome P-450. Isolation and purification of the protein and cloning and sequencing of its operon. J Biol Chem 267: 14193–14203 (1992).
Pietrusko R: Biochemistry and Physiology of Substance Abuse Vol. 1, pp. 89–127 CRC Press, Boca Raton, FL (1989).
Rongnoparut P, Weaver S: Isolation and characterization of a cytosolic aldehyde dehydrogenase-encoding cDNA from mouse liver. Gene 101: 261–265 (1991).
Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1989).
Skiver K, Mundy J: Gene expression in response to abscisic acid and osmotic stress. Plant Cell 2: 503–512 (1990).
Turner NC: Drought resistance and adaptation to water deficits in crop plants. In: Mussell H, Staples RC (eds) Stress Physiology in Crop Plants, pp. 181–194. Wiley-Interscience, New York (1979).
Von Bahr-Lindstrom H, Hempel J, Joernvall H: The cytoplasmic isoenzyme of horse liver aldehyde dehydrogenase. Relationship to the corresponding human isoenzyme. Eur J Biochem 141: 37–42 (1984).
Weretilnyk EA, Bednarek S, McCue KF, Rhodes D, Hanson AD: Comparative biochemical and immunological studies of the glycine betaine synthesis pathway in diverse families of dicotyledons. Planta 178: 342–352 (1989).
Weretilnyk EA, Hanson AD: Molecular cloning of a plant betaine-aldehyde dehydrogenase, an enzyme implicated in adaptation to salinity and drought. Proc Natl Acad Sci USA 87: 2745–2749 (1990).
Weretilnyk E, Orr W, White TC, Iu B, Singh J: Characterization of three related low-temperature-regulated cDNAs from winter Brassica napus. Plant Physiol 101: 171–177 (1993).
Yamaguchi-Shinozaki K, Koizumi M, Urao S, Shinozaki K: Molecular cloning and characterization of 9 cDNAs for genes that are responsive to desiccation in Arabidopsis thaliana: sequence analysis of one cDNA clone that encodes a putative transmembrane channel protein. Plant Cell Physiol 33: 217–224 (1992).
Yamaguchi-Shinozaki K, Shinozaki K: The plant hormone abscisic acid mediates the drought-induced expression but not the seed-specific expression of rd22, a gene responsive to dehydration stress in Arabidopsis thaliana. Mol Gen Genet 238: 17–25 (1993).
Yamaguchi-Shinozaki K, Shinozaki K: A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 6: 251–264 (1994).
Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN: Living with water stress: evolution of osmolyte systems. Science 217: 1214–1222 (1982).
Yeh K-W, Juang R-H, Su J-C: A rapid and efficient method for RNA isolation from plants with high carbohydrate content. Focus 13: 102–103 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stroeher, V.L., Boothe, J.G. & Good, A.G. Molecular cloning and expression of a turgor-responsive gene in Brassica napus . Plant Mol Biol 27, 541–551 (1995). https://doi.org/10.1007/BF00019320
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00019320