Abstract
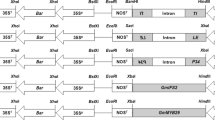
Inheritance of foreign genes neo and gusA in rice (Oryza sativa L. cv. IR54 and Radon) has been investigated in three different primary (T0) transformants and their progeny plants. T0 plants were obtained by co-transforming protoplasts from two different rice suspension cultures with the neomycin phosphotransferase II gene [neo or aph (3′) II] and the β-glucuronidase gene (uidA or gusA) residing on separate chimeric plasmid constructs. The suspension cultures were derived from callus of immature embryos of indica variety IR54 and japonica variety Radon. One transgenic line of Radon (AR2) contained neo driven by the CaMV 35S promoter and gusA driven by the rice actin promoter. A second Radon line (R3) contained neo driven by the CaMV 35S promoter and gusA driven by a promoter of the rice tungro bacilliform virus. The third transgenic line, IR54-1, contained neo driven by the CaMV 35S promoter and gusA driven by the CaMV 35S.
Inheritance of the transgenes in progeny of the transgenic rice was investigated by Southern blot analysis and enzyme assays. Southern blot analysis of genomic DNA showed that, regardless of copy numbers of the transgenes in the plant genome and the fact that the two transgenes resided on two different plasmids before transformation, the introduced gusA and neo genes were stably transmitted from one generation to another and co-inherited together in transgenic rice progeny plants derived from self-pollination. Analysis of GUS and NPT II activities in T1 to T2 plants provided evidence that inheritance of the gusA and neo genes was in a Mendelian fashion in one plant line (AR2), and in an irregular fashion in the two other plant lines (R3 and IR54-1). Homozygous progeny plants expressing the gusA and neo genes were obtained in the T2 generation of AR2, but the homozygous state was not found in the other two lines of transgenic rice.
Similar content being viewed by others
References
Bhattacharyya-Pakrasi M, Peng J, Elmer JS, Laco G, Shen P, Kaniewska MB, Kononowicz H, Wen F, Hodges TK, Beachy RN: Expression of a promoter from the Rice Tungro Bacilliform Virus. Plant J 4: 71–79 (1993).
Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 (1976).
Budar F, Thia-Toong L, Von Montagu M, Hernalsteens J-P. Agrobacterium-mediated gene transfer results mainly in transgenic plants transmitting T-DNA as a single Mendelian factor. Genetics 114: 303–313 (1986).
Czernilofsky AP, Hain R, Herrera-Estrella L, Lorz H, Goyvaerts E, Baker BJ, Schell J: Fate of selectable marker DNA integrated into the genome of Nicotiana tabacum. DNA 5: 101–113 (1986).
Christou P, Ford TL, Kofron M: Production of transgenic rice (Oryza sativa) from agronomically important Indica and Japonica varieties via electric discharge particle acceleration of exogenous DNA into immature zygotic embryos. Bio/technology 9: 957–962 (1991).
Christou P, Swain WF, Yang NS, McCabe DE: Inheritance and expression of foreign genes in transgenic soybean plants. Proc Natl Acad Sci USA 86: 7500–7504 (1989).
Datta SK, Peterhans A, Datta K, Potrykus I: Genetically engineered fertile indica-rice recovered from protoplasts. Bio/technology 8: 736–740 (1990).
De Carvalho F, Gheysen G, Kushnir S, Von Montagu M, Inze D, Castresana C: Suppression of β-1,3-glucanase transgene expression in homozygous plants. EMBO J 11: 2595–2602 (1992).
De Block M, Herrera-Estrelle L, Von Montagu M, Schell J, Zambryski P: Expression of foreign genes in regenerated plants and their progeny. EMBO J 3: 1681–1689 (1989).
Deroles SC, Gardner RC: Expression and inheritance of kanamycin resistance in a large number of transgenic petunias generated by Agrobacterium-mediated transformation. Plant Mol Biol 11: 355–364 (1988).
Feldmann KA, Marks MD: Agrobacterium-mediated transformation of germinating seeds of Arabidopsis thaliana: a non-tissue culture approach. Mol Gen Genet 208: 1–9 (1987).
Folger KR, Wong A, Wahl G, Capecchi MR: Patterns of integration of DNA microinjected into cultured mammalian cells: evidence for homologous recombination between injected plasmid DNA molecules. Mol Cell Biol 2: 1372–1387 (1982).
Fromm ME, Morrish F, Armstrong C, Williams R, Thomas J, Klein TM: Inheritance and expression of chimeric genes in the progeny of transgenic maize plants. Bio/technology 8: 833–839 (1990).
Gordon-Kamm WJ, Spencer TM, Mangano ML, Adams TR, Daines RJ, Start WG, O'Brien JV, Chambers SA, Adams WR, Willetts NG, Rice TB, Macky CJ, Krueger RW, Kausch AP, Lemaux PG: Transformation of maize cells and regeneration of fertile transgenic plants. Plant Cell 2: 603–618 (1990).
Goto F, Toki S, Uchimiya H: Inheritance of a co-transferred foreign gene in the progenies of transgenic rice plants. Transgenic Res 2: 302–305 (1993).
Hain R, Stabel P, Czernilfsky AP, Steinbiß HH, Herrera-Estrella L, Schell J: Uptake, integration, expression and genetic transmission of a selectable chimaeric gene by plant protoplasts. Mol Gen Genet 199: 161–168 (1985).
Horsch RB, Fraley RT, Rogers SG, Sanders PR, Lloyd A, Hoffmann N: Inheritance of functional foreign genus in plants. Science 223: 496–498 (1984).
Jefferson RA: Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5: 387–405 (1987).
Katz KS, Ratner DI: Homologous recombination and the repair of double-strand breaks during cotransformation of Dictyostelium discoideum. Mol Cell Biol 8: 2779–2786 (1988).
Lyznik LA, Ryan RA, Ritchie SW, Hodges TK: Stable co-transformation of maize protoplasts with gusA and neo genes. Plant Mol Biol 13: 151–161 (1989).
Maniatis T, Fritsch EF, Sambrook J: Molecular Cloning: A Laboratory Manual, pp. 9.34–9.40, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY (1982).
McCouch SR, Kochert G, Yu ZH, Wang ZY, Khush GS, Coffman WR, Tanksley SD: Molecular mapping of rice genome. Theor Appl Genet 76: 815–829 (1988).
McElroy D, Zhang W, Cao J, Wu R: Isolation of an efficient actin promoter for use in rice transformation. Plant Cell 2: 163–171 (1990).
Muller AJ, Mendel RR, Schiemann J, Simoens C, Inze D: High meiotic stability of a foreign gene introduced in to tobacco by Agrobacterium-mediated transformation. Mol Gen Genet 207: 171–175 (1987).
Otten L, Hernalsteens JP, Von Montagu M, Schieder O, Straub J, Schell J: Mendelian transmission of genes introduced into plants by the Ti plasmids of Agrobacterium tumefaciens. Mol Gen Genet 183: 209–213 (1981).
Peng J, Lyznik LA, Lee L, Hodges TK: Co-transformation of indica rice protoplasts with gusA and neo genes. Plant Cell Rep 9: 168–172 (1990).
Peng J, Kononowicz H, Hodges TK: Transgenic indica rice plants. Theor Appl Genet 83: 855–863 (1992).
Peng J, Wen F, Hodges TK: A simplified method for qualitatively assaying both glucuronidase and neomycin phosphorase II activities in transgenic plants. Plant Mol Biol Rept 10: 38–47 (1993).
Potrykus I, Paszkowski J, Saul MW, Petruska J, Sillito RD: Molecular and general genetics of a hybrid foreign gene introduced into tobacco by direct gene transfer. Mol Gen Genet 199: 169–171 (1985).
Rathore KS, Chowdhury VK, Hodges TK: Use of bar as a selectable marker gene for the production of herbicideresistant rice plants from protoplasts. Plant Mol Biol 21: 871–884 (1993).
Rhodes CA, Pierce DA, Mettler LT, Mascarenhas D, Detmer JJ: Genetically transformed maize plants from protoplasts. Science 240: 204–207 (1988).
Shimamoto K, Terada R, Izawa T, Fujimoto H: Fertile transgenic rice plants regenerated from transformed protoplasts. Nature 338: 274–276 (1989).
Spencer TM, O'Brien JV, Start WG, Adams TR: Segregation of transgenes in maize. Plant Mol Biol 18: 201–210 (1992).
Strickberger MW: Genetics, 2nd ed. Macmillan, New York (1976).
Tomes DT, Weissinger AK, Ross M, Higgins R, Drummond BJ, Schaaf S, Malone-Schoneberg JB, Stabell M, Flynn P, Anderson J, Howard J: Transgenic tobacco plants and their progeny derived by microprojectile bombardment of tobacco leaves. Plant Mol Biol 14: 261–268 (1990).
Vasil V, Castillo AM, Fromm ME, Vasil IK: Herbicide resistant fertile transgenic wheat plants obtained by microprojectile bombardment of regenerable embryogenic callus. Bio/technology 10: 667–674 (1992).
Wen F, Peng J, Lister RM, Hodges TK: A procedure for regenerating japonica and indica varieties of rice from protoplasts. Plant Mol Biol Rep 9: 308–321 (1992).
Zhang W, McElroy D, Wu R: Analysis of rice Act1 5′ region activity in transgenic rice plants. Plant Cell 3: 1155–1165 (1991).
Zhang W, Wu R: Efficient regeneration of transgenic plants from rice protoplasts and correctly regulated expression of the foreign gene in the plants. Theor Appl Genet 76: 835–840 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Peng, J., Wen, F., Lister, R.L. et al. Inheritance of gusA and neo genes in transgenic rice. Plant Mol Biol 27, 91–104 (1995). https://doi.org/10.1007/BF00019181
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00019181