Abstract
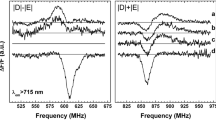
Fluorescence Detected Magnetic Resonance (FDMR) spectra have been measured for whole cells and isolated chlorosomal fractions for the green photosyntheic bacteria Chlorobium phaeobacteroides (containing bacteriochlorophyll e, and isorenieratene as major carotenoid) and Chlorobium limicola (containing bacteriochlorophyll c, and chlorobactene as major carotenoid). The observed transition at 237 MHz (identical in both bacteria) and > 1100 MHz can be assigned, by analogy with published data on other carotenoids, to the 2E and D + E transitions, respectively, of Chlorobium carotenoids. Their zero field splitting (ZFS) parameters are estimated to be: |D|=0.0332 cm−1 and |E|=0.0039 cm−1 (chlorobactene), and |D|=0.0355 cm−1 and |E|=0.0039 cm−1 (isorenieratene). In the intermediate frequency range 300–1000 MHz the observed transitions can be assigned to chlorosomal bacteriochlorophylls c and e, and to bacteriochlorophyll a located in the chlorosome envelope and water-soluble protein. The bacteriochlorophyll e triplet state measured in 750 nm fluorescence (aggregated chlorosomal BChl e) is characterised by the ZFS parameters: |D|=0.0251 cm−1 and |E|=0.0050 cm−1.
Similar content being viewed by others
Abbreviations
- BChl -:
-
bacteriochlorophyll
- BPh -:
-
bacteriopheophytin
- Chl. -:
-
Chlorobium
- F(A)(O)DMR -:
-
fluorescence (absorption) (optical) detected magnetic resonance
- FF -:
-
fluorescence fading
- ISC -:
-
intramolecular intersystem crossing
- RC -:
-
reaction center
- ZFS -:
-
zero field splitting
References
Angerhofer A, VonSchütz JU and Wolf HC (1984) Fluorescence-ODMR of reaction centers of Rhodopseudomonas viridis. Z Naturforsch 39c: 1085–1090
Aust V, Angerhofer A, Ullrich J, VonSchütz JU, Wolf HC and Codgell RJ (1991) ADMR of carotenoid triplet states in bacterial photosynthetic antenna and reaction center complexes. Chem Phys Lett 181: 213–221
Blankenship RE, Cheng P, Causgrove PT, Brune DC, Wang SH, Chon J and Wang J (1993) Redox regulation of energy transfer efficiency in antennas of green photosynthetic bacteria. Photochem Photobiol 37: 103–107
Brune DC, Nozawa T and Blankenship RE (1987) Antenna organization in green photosynthetic bacteria. 1. Oligomeric bacteriochlorophyll c as a model for the 740 nm absorbing bacteriochlorophyll c in Chloroflexus aurantiacus chlorosomes. Biochem 26: 8644–8652
Carbonera D, Giacometti G, Agostini G, Angerhofer A and Aust V (1992) ODMR of carotenoid and chlorophyll triplets in CP43 and CP47 complexes of spinach. Chem Phys Lett 194: 275–281
Frank HA (1992) Electron paramagnetic resonance studies of carotenoids. In: Packer L (ed) Methods in Enzymology, Vol 213, pp 305–312. Academic Press, San Diego
Frick J, VonSchütz JU, Wolf HC and Kothe G (1990) First detection of the (non-phosphorescent) triplet state in single crystals of β-carotene. Mol Cryst Liq Cryst 183: 269–272
Gerola PD and Olson JM (1986) A new bacteriochlorophyll α-protein complex associated with the chlorosomes of green sulfur bacteria. Biochim Biophys Acta 848: 69–70
Gerola PD, Højrup P, Kundsen J, Roepstorff P and Olson JM (1988) The bacteriochlorophyll c-binding protein from chlorosomes of Chlorobium limicola f. thiosulfatophilum. In: Olson JM (ed) Green Photosynthetic Bacteria, pp 43–53. Plenum Press, New York
Gorlenko VM (1988) Ecological niches of green sulfur bacteria. In: Olson JM (ed) Green Photosynthetic Bacteria, pp 257–267. Plenum Press, New York
Griebenow K and Holzwarth AR (1989) Pigment organization and energy transfer in green bacteria. 1. Isolation of native chlorosomes free of bound bacteriochlorophyll a from Chloroflexus aurantiacus by gel-electrophoretic filtration. Biochim Biophys Acta 973: 235–240
Hála J, Searle GFW, Schaafsma TJ, VanHoek A, Pancoska P, Blaha K and Vacek K (1986) Picosecond laser spectroscopy and optically detected magnetic resonance studies on a model photosynthetic system. Photochem Photobiol 44: 527–534
Hoff AJ (1986) Optically detected magnetic resonance (ODMR) of triplet states in vivo. In: Staehelin LA and Arntzen CJ (eds) Photosynthesis III (Encyclopedia of Plant Physiology), pp 400–421. Springer-Verlag, Berlin
Kramer HJM, Kingma H, Swarthoff T and Amesz J (1982) Prompt and delayed fluorescence in pigment-protein complexes of a green photosynthetic bacterium. Biochim Biophys Acta 681: 359–364
Matsuura K and Olson JM (1990) Reversible conversion of aggregated bacteriochlorophyll c to the monomeric form by 1-hexanol in chlorosomes from Chlorobium and Chloroflexus. Biochim Biophys Acta 1019: 233–238
Nitschke W, Feiler U and Rutherford AW (1990) Photosynthetic reaction centers of green sulfur bacteria studied by EPR. Biochemistry 29: 3834–3842
Olson JM (1980) Chlorophyll organization in green photosynthetic bacteria. Biochim Biophys Acta 594: 33–51
Olson JM and Pedersen JP (1988) Bacteriochlorophyll c aggregates in carbon tetrachloride as models for chlorophyll organization in green photosynthetic bacteria. In: Scheer H and Schneider S (eds) Photosynthetic Light-Harvesting Systems, pp 365–373. Walter de Gruyter, Berlin
Ormerod JG (1992) Physiology of the photosynthetic prokaryotes. In: Mann NH and Noel GC (eds) Photosynthetic Prokaryotes, pp 93–120. Plenum Press, New York
Otte SCM, Van DerHeiden JC, Pfennig N and Amesz J (1991) A comparitive study of the optical characteristics of intact cells of photosynthetic green sulfur bacteria containing bacteriochlorophyll c, d or e. Photosynth Res 28: 77–87
Ros M and Groenen EJJ (1991) An electron-spin-echo study of the non-radiative triplet state of polyenals. J Chem Phys 94: 7640–7648
Schmidt K (1978) Biosynthesis of carotenoids. In: Clayton RK and Sistrom WR (eds) The Photosynthetic Bacteria, pp 729–750. Plenum Press, New York
Searle GFW and Schaafsma TJ (1992) Fluorescence detected magnetic resonance of the primary donor and inner core antenna chlorophyll in Photosystem I reaction centre protein: Sign inversion and energy transfer. Photosynth Res 32: 193–206
Searle GFW, VanHoek A and Schaafsma TJ (1983) Picosecond laser spectroscopy and magnetic resonance studies on isolated chlorophyll-proteins. In: Doust TAM and West MA (eds) Picosecond Chemistry and Biology, pp 35–67. Science Reviews Ltd, Northwood, Middlesex, England
Thurnauer MC and Norris JR (1977) The ordering of the zero field triplet spin sublevels in the chlorophylls. A magnetophotoselection study. Chem Phys Lett 47: 100–105
Uehara K and Olson JM (1992) Aggregation of bacteriochlorophyll c homologs to dimers, tetramers and polymers in water-saturated carbon tetrachloride. Photosynth Res 33: 251–257
Van DerVos R, Carbonera D and Hoff AJ (1991) Microwave and optical spectroscopy of carotenoid triplets in light-harvesting complex LHCII of spinach. Appl Mag Res 2: 179–202
VanDorp WG, Schaafsma TJ, Soma M and Van DerWaals JH (1973) Investigation of the lowest triplet state of free base porphin by microwave induced changes in its fluorescence. Chem Phys Lett 21: 221–225
VanDorssen RJ, Gerola PD, Olson JM and Amesz J (1986) Optical and structural properties of chlorosomes of the photosynthetic green sulfur bacterium Chlorobium limicola. Biochim Biophys Acta 848: 77–82
Vasmel H, DenBlanken HJ, Dijkman JT, Hoff AJ and Amesz J (1984) Triplet-minus-singlet absorbance difference spectra of reaction centers and antenna pigments of the green photosynthetic bacterium Prosthecochloris aestuarii. Biochim Biophys Acta 767: 200–208
Wang J, Brune DC and Blankenship RE (1990) Effects of oxidants and reductants on the efficiency of excitation transfer in green photosynthetic bacteria. Biochim Biophys Acta 1015: 457–463
Zuber H and Brunisholz RA (1991) Structure and function of antenna polypeptides and chlorophyll-protein complexes: principles and variability. In: Scheer H (ed) Chlorophylls, pp 627–703. CRC, Boca Raton, USA
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Psencík, J., Searle, G.F.W., Hála, J. et al. Fluorescence detected magnetic resonance (FDMR) of green sulfur photosynthetic bacteria Chlorobium sp.. Photosynth Res 40, 1–10 (1994). https://doi.org/10.1007/BF00019040
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00019040