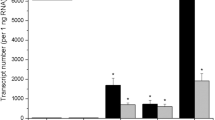
Abstract
Patatin is a family of lipid acyl hydrolases that accounts for 30 to 40% of the total soluble protein in potato (Solanum tuberosum L.) tubers. To examine the regulation of the patatin genes, we constructed a chimeric gene containing 2.5 kb of 5′ flanking sequence from the class I patatin genomic clone PS20 transcriptionally fused to β-glucuronidase (GUS) and introduced it into potato plants using an Agrobacterium tumefaciens Tiplasmid vector. While the chimeric gene was expressed at high levels in tubers and in stolons attached to developing tubers, it was not normally expressed in leaves, stems, roots, or in stolons before tuberizatization. However, the expression of the class I patatin-GUS construct was not “tuber-specific” since leaf and stem explants cultured on medium containing 300 to 400 mM sucrose showed GUS activity equal or greater than that of tubers. The sucrose induction of GUS activity in leaf and stem explants was accompanied by the accumulation of patatin protein and large amounts of starch, but not by the morphological changes that normally are associated with tuberization. In contrast, the GUS reporter gene under the control of the 35S promoter of cauliflower mosaic virus showed an essentially uniform pattern of expression in transgenic potato plants and was not induced by sucrose.
Similar content being viewed by others
References
Andrews DL, Beames B, Summers MD, Park WD: Characterization of the lipid acyl hydrolase activity of the major potato (Solanum tuberosum) tuber protein, patatin, by cloning and abundant expression in a baculovirus vector. Biochem J 252: 199–206 (1988).
Bevan M: Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res 12: 8711–8721 (1984).
Bevan M, Barker R, Goldsbrough A, Jarvis M, Kavanagh T, Iturriaga G: The structure and transcription start site of a major tuber protein patatin. Nucleic Acids Res 14: 5564–5566 (1986).
Bourque JE, Miller JC, Park WD: Use of an in vitro tuberization system to study tuber protein gene expression. In Vitro Cell Dev Biol 23: 381–386 (1987).
Bradford M: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72: 248–254 (1976).
Cutter EG: Structure and development of the potato plant. In: Harris PM (ed) The Potato Crop: The Scientific Basis for Improvement, pp. 70–152. Chapman and Hall, London (1978).
Ewing EE: Cuttings as simplified models of the potato plant. In: Li PH (ed) Potato Physiology, pp. 153–207. Academic Press, Orlando (1985).
Ewing EE, Wareing PF: Heat stress and the tuberization stimulus. Am Potato J 58: 31–49 (1981).
Horsch RB, Fry JB, Hoffmann NL, Wallroth M, Eichholtz D, Rogers SB, Fraley RT: A simple and general method for transferring genes into plants. Science 227: 1229–1231 (1985).
Jefferson RA, Kavanagh TA, Bevan MW: GUS fusion: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 (1987).
Logemann J, Mayer JE, Schell J, Willmitzer L: Differential expression of genes in potato tubers after wounding. Proc Natl Acad Sci USA 85: 1136–1140 (1988).
Mignery CA, Pikaard CS, Park WD: Molecular characterization of the patatin multigene family of potato. Gene 62: 27–44 (1988).
Murashige T, Skoog F: A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 (1962).
Oparka KJ, Wright KM: Osmotic regulation of starch synthesis in potato tubers? Planta 174: 123–126 (1988).
Paiva EP, Lister RM, Park WD: Induction and accumulation of the major potato tuber protein, patatin. Plant Physiol 71: 161–168 (1983).
Pikaard CS, Mignery GA, Ma DP, Stark VJ, Park WD: Sequence of two apparent pseudogenes of the major tuber protein patatin. Nucleic Acids Res 14: 5564–5566 (1986).
Racusen D: Occurrence of patatin during growth and storage of potato tubers. Can J Bot 61: 370–373 (1983).
Racusen D, Foote M: A major soluble glycoprotein of potato. J Food Biochem 4: 43–52 (1980).
Rosahl S, Schmidt R, Schell J, Willmitzer L: Isolation and characterization of a gene from Solanum tuberosum encoding patatin, the major storage protein of potato tubers. Mol Gen Genet 203: 214–220 (1986).
Twell D, Ooms G: Differential and photomorphogenic regulation of a chimaeric class II patatin gene in transgenic potato. Nucleic Acids Res (in press).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wenzler, H.C., Mignery, G.A., Fisher, L.M. et al. Analysis of a chimeric class-I patatin-GUS gene in transgenic potato plants: High-level expression in tubers and sucrose-inducible expression in cultured leaf and stem explants. Plant Mol Biol 12, 41–50 (1989). https://doi.org/10.1007/BF00017446
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00017446