Abstract
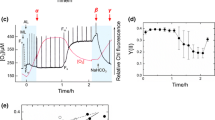
Light-activated hydrogen and oxygen evolution as a function of CO2 concentration in helium were measured for the unicellular green alga Chlamydomonas reinhardtii. The concentrations were 58, 30, 0.8 and 0 ppm CO2. The objective of these experiments was to study the differential affinity of CO2/HCO -3 for their respective Photosystem II and Calvin cycle binding sites vis-à-vis photoevolution of molecular oxygen and the competitive pathways of hydrogen photoevolution and CO2 photoassimilation. The maximum rate of hydrogen evolution occurred at 0.8 ppm CO2, whereas the maximum rate of oxygen evolution occurred at 58 ppm CO2. The key result of this work is that the rate of photosynthetic hydrogen evolution can be increased by, at least partially, satisfying the Photosystem II CO2/HCO -3 binding site requirement without fully activating the Calvin-Benson CO2 reduction pathway. Data are presented which plot the rates of hydrogen and oxygen evolution as functions of atmospheric CO2 concentration in helium and light intensity. The stoichiometric ratio of hydrogen to oxygen changed from 0.1 at 58 ppm to approximately 2.5 at 0.8 ppm. A discussion of partitioning of photosynthetic reductant between the hydrogen/hydrogenase and Calvin-Benson cycle pathways is presented.
Similar content being viewed by others
Abbreviations
- PET:
-
photosynthetic electron transport
- PS:
-
Photosystem
References
Baker W, Combs FJ, Zinn TL, Wotring AW and Wall RF (1956) The galvanic cell oxygen analyzer. Ind Eng Chem 51: 727–730
Bishop NI, Frick M and Jones LW (1977) Photohydrogen production in green algae: Water serves as the primary substrate for hydrogen and oxygen production. In: Mitsui A, Miyachi S, San Pietro A and Tamura S (eds) Biological Solar Energy Conversion, pp 3–22. Academic Press, New York
Bruinsma J (1961) A comment on the spectrophotometric determination of chlorophyll. Biochim Biophys Acta 52: 576–578
Eaton-Rye JJ and Govindjee (1984) A study of the specific effect of bicarbonate on photosynthetic electron transport in the presence of methyl viologen. Photobiochem Photobiophys 8: 279–288
Gaffron H and Rubin J (1942) Fermentative and photochemical production of hydrogen in algae. J Gen Physiol 26: 219–240
Graves DA, Reeves ME and Greenbaum E (1988) Establishment of control parameters for in situ, automated screening of sustained hydrogen photoproduction by individual algal colonies. Plant Physiol 87: 603–608
Graves DA, Tevault CV and Greenbaum E (1989) Control of photosynthetic reductant: The role of light and temperature on sustained hydrogen photoevolution by Chlamydomonas sp. in an anoxic, carbon dioxide-containing atmosphere. Photochem Photobiol 50: 571–576
Greenbaum E (1979) The turnover times and pool sizes of photosynthetic hydrogen production by green algae. Sol Energ 23: 315–320
Greenbaum E (1984) Biophotolysis of water: The light saturation curves. Photobiochem Photobiophys 8: 323–332
Greenbaum E (1988a) Hydrogen production by algal water splitting. In: Lembi CA and Waaland JR (eds) Algae and Human Affairs, pp 284–300. Cambridge University Press, Cambridge, England
Greenbaum E (1988b) Energetic efficiency of hydrogen photoevolution by algal water splitting. Biophys J 54: 365–368
Jursinic PA and Stemler A (1992) High rates of Photosystem II electron flow occur in maize thylakoids when the high-affinity binding site for bicarbonate is empty of all monovalent anions or has bicarbonate bound. Biochem Biophys Acta 1098: 359–367
Klein U and Betz A (1978) Fermentative metabolism of hydrogen evolving Chlamydomonas moewussi. Plant Physiol 61: 953–956
Lorimer GH, Badger MR and Andrews TJ (1976) The activation of ribulose-1,5-bisphosphate carboxylase by carbon dioxide and magnesium ions. Equilibria, kinetics, a suggested mechanism and physiological implications. Biochem 15: 529–536
Reeves M and Greenbaum E (1985) Long-term endurance and selection studies in hydrogen and oxygen photoproduction by Chlamydomonas reinhardtii. Enzyme Microb Technol 7: 169–174
Stemler A (1982) The functional role of bicarbonate in photosynthetic light reaction II. In: Govindjee (ed) Photosynthesis, Vol II, pp 513–538. Academic Press, New York
Stemler A and Govindjee (1973) Bicarbonate ion as a critical factor in photosynthetic oxygen evolution. Plant Physiol 52: 119–123
Vermaas WFJ and Govindjee (1982) Bicarbonate or carbon dioxide as a requirement for efficient electron transport on the acceptor side of Photosystem II. In: Govindjee (ed) Photosynthesis, Vol II, pp 543–556. Academic Press, New York
Warburg O and Krippahl G (1960) Notwendigkeit der Kohlensäure für die Chinon- und Ferricyanid-Reaktionen in grünen Grana. Z Naturforsch, B, 15B: 367–369
Xu C, Taoka S, Crofts AR and Govindjee (1991) Kinetic characteristics of formate/formic acid binding at the plastoquinone reductase site in spinach thylakoids. Biochim Biophys Acta 1098: 32–40
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cinco, R.M., MacInnis, J.M. & Greenbaum, E. The role of carbon dioxide in light-activated hydrogen production by Chlamydomonas reinhardtii . Photosynth Res 38, 27–33 (1993). https://doi.org/10.1007/BF00015058
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00015058