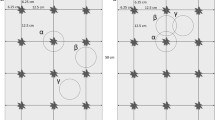
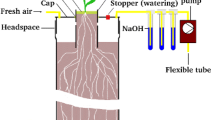
Abstract
A model rhizodeposition technique to estimate the root and microbial components of 14C soil/root respiration in pulse-labelling experiments is described. The method involves the injection of model rhizodeposits, consisting of 14C-labelled glucose, root extract or root cell wall material, into the rooted soil of an unlabelled plant, simultaneously with the pulse-labelling of a separate but similar plant with 14CO2. In a growth chamber experiment with 30 day old wheat and barley the contribution of direct root respiration to 14C soil/root respiration over a 26 day period after labelling was estimated 89–95%. Estimates of direct root respiration in field-grown wheat and barley at different development stages in most cases accounted for at least 75% of 14C soil/root respiration over a 21 day period after labelling. The mineralization rate of injected 14C-glucose was positively correlated with the concentration of glucose-C established in soil. The use of the method in rhizosphere carbon budget estimations is evaluated.
Similar content being viewed by others
References
Amato M 1983 Determination of carbon 12C and 14C in plant and soil. Soil Biol. Biochem. 15, 611–612.
Anderson J P E 1982 Soil Respiration. In Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties, Second Edition. Eds. A LPage, R HMiller and D RKeeney. pp 852–871. Am. Soc. Agron. Inc., Soil Sci. Soc. Am. Inc., Madison, Wisconsin, USA.
Barber D A and Martin J K 1976 The release of organic substances by cereal roots into soil. New Phytol. 76, 69–80.
Bremer E and Kuikman P J 1994 Microbial utilization of 14C[U]glucose in soil over a wide range of glucose addition rates. Soil Biol. Biochem. 26, 511–517.
Bremer E and VanKessel C 1990 Extractability of microbial 14C and 15N following addition of variable rates of labelled glucose and (NH4)2SO4 to soil. Soil Biol. Biochem. 22, 707–713.
Cheng W, Coleman D C, Carroll C R and Hoffman C A 1993 In situ measurement of root respiration and soluble C concentrations in the rnizosphere. Soil Biol. Biochem. 25, 1189–1196.
Clarholm M 1985 Possible roles for roots, bacteria, protozoa and fungi in supplying nitrogen to plants. In Ecological Interactions in Soil. Plants, Microbes an Animals. Ed. A HFitter. pp 355–365. Blackwell Scientific Publications, Oxford, UK.
Darrah P R 1991 Models of the rhizosphere I. Microbial population dynamics around a root releasing soluble and insoluble carbon. Plant and Soil 133, 187–199.
Deacon J W and Lewis S J 1982 Natural senescence of the root cortex of spring wheat in relation to susceptibility to common root rot (Cochliobolus sativus) and growth of a free-living nitrogen-fixing bacterium. Plant and Soil 66, 13–20.
Fao-Unesco 1981 Soil Map of the World, Volume V, Europe. Unesco, Paris, France. 199 p.
Gordon A J, Ryle G J A and Powell C E 1977 The strategy of carbon utilization in uniculm barley I. The chemical fate of photosynthetically assimilated 14C. J. Exp. Bot. 28, 1258–1269.
Helal H M and Sauerbeck D 1991 Short-term determination of the actual respiration rate of intact plant roots. In Plant Roots and Their Environment. Eds. B LMcMichael and HPersson. pp 88–92. Elsevier, Amsterdam, The Netherlands.
Holden J 1975 Use of nuclear staining to assess rates of cell death in cortices of cereal roots. Soil Biol. Biochem. 7, 333–334.
Johansson G 1992 Release of organic C from growing roots of meadow fescue (Festuca pratensis L.). Soil Biol. Biochem. 24, 427–433.
Johnen B G and Sauerbeck D R 1977 A tracer technique for measuring growth, mass and microbial breakdown of plant roots during vegetation. In Soil Organisms as Components of Ecosystems. Eds. V Lohm and T Persson. pp 366–373. Ecological Bulletins 25, Stockholm, Sweden.
Kooistra M J, Lebbink G and Brussaard L 1989 The Dutch programme on soil ecology of arable farming systems. 2. Geogenesis, agricultural history, field site characteristics and present farming systems at the Lovinkhoeve experimental farm. Agric. Ecosystems Environ. 27, 361–387.
Lekkerkerk L J A, Van DeGeijn S C and VanVeen J A 1990 Effects of elevated atmospheric CO2-levels on the carbon economy of a soil planted with wheat. In Soils and the Greenhouse Effect. Ed. A FBouwman. pp 423–429. Wiley and Sons, Chichester, UK.
Liljeroth E, VanVeen J A and Miller H J 1990 Assimilate translocation to the rhizosphere of two wheat cultivars and subsequent utilization by microorganisms at two soil nitrogen levels. Soil Biol. Biochem. 22, 1015–1021.
Lynch J M and Whipps J M 1990 Substrate flow in the rhizosphere. Plant and Soil 129, 1–10.
Martens R 1982 Apparatus to study the quantitative relationships between root exudates and microbial populations in the rhizosphere. Soil Biol. Biochem. 14, 315–317.
Martens R 1985 Limitations in the application of the fumigation technique for biomass estimations in amended soils. Soil Biol. Biochem. 17, 57–63.
Meharg A A and Killham K 1990 Carbon distribution within the plant and rhizosphere in laboratory and field-grown Lolium perenne at different stages of development. Soil Biol. Biochem. 22, 471–477.
Merckx R, DenHartog A and VanVeen J A 1985 Turnover of root-derived material and related microbial biomass formation in soils of different texture. Soil Biol. Biochem., 17, 565–569.
Merckx R, Dijkstra A, DenHartog A and VanVeen J A 1987 Production of root-derived material and associated microbial growth in soil at different nutrient levels. Biol. Fertil. Soils 5, 126–132.
Minchin P E H and McNaughton G S 1984 Exudation of recently fixed carbon by non-sterile roots. J. Exp. Bot. 35, 74–82.
Newman E I 1985 The rhizosphere: carbon sources and microbial populations. In Ecological Interactions in Soil. Plants, Microbes and Animals. Ed. A HFitter. pp 107–121. Blackwell Scientific Publications, Oxford, UK.
Rovira A D 1973 Zones of exudation along plant roots and spatial distribution of micro-organisms in the rhizosphere. Pestic. Sci. 4, 361–366.
Sparling G P and West A W 1988 A direct extraction method to estimate soil microbial C: calibration in situ using microbial respiration and 14C labelled cells. Soil Biol. Biochem. 20, 337–343.
Sparling G P, Ord B G and Vaughan D 1981 Microbial biomass and activity in soils amended with glucose. Soil Biol. Biochem. 13, 99–104.
Swinnen J 1994 Rhizodeposition and turnover of root-derived organic material in barley and wheat under conventional and integrated management. Agric. Ecosystems Environ. (In press).
Swinnen J and VanVeen J A 1993 Unterscheidung von Wurzel- und microbieller Atmung im Boden durch Exudatmarkierung. In Bodennutzung und Bodenfruchtbarkeit, Vol. 4. Humushaushalt. Berichte über Landwirtschaft 206. Sonderh. pp 114–116. Parey, Hamburg, Berlin.
Swinnen J, VanVeen J A and Merckx R 1994a 14C pulse-labelling of field-grown spring wheat: an evaluation of its use in rhizosphere carbon budget estimations. Soil Biol. Biochem. 26, 161–170.
Swinnen J, VanVeen J A and Merckx R 1994b Rhizosphere carbon fluxes in field-grown spring wheat: model calculations based on 14C partitioning after pulse-labelling. Soil Biol. Biochem. 26, 171–182.
Trofymow J A, Coleman D C and Cambardella C 1987 Rates of rhizodoposition and ammonium depletion in the rhizosphere of axenic oat roots. Plant and Soil 97, 333–344.
Van Noordwijk M and Van De Geijn S C 1994 Root, shoot and soil parameters required for process-oriented models of crop growth limited by water or nutrients. In New Methods of Root Research. Ed. A Smucker. A.S.A. Special Publication. (In press).
VanVeen J A, Merckx R and Van DeGeijn S C 1989 Plant- and soil related controls of the flow of carbon from roots through the soil microbial biomass. Plant and Soil 116, 167–175.
Warembourg F R 1975a Application de techniques radioisotopiques à l'étude de l'activité biologique dans la rhizosphère des plantes. Rev. Écol. Biol. Sol 12, 261–272.
Warembourg F R 1975b Le degagement de CO2 dans la rhizosphère des plantes. Soc. Bot. Fr., Coll. Rhizosphère, 77–87.
Warembourg F R and Billes G 1979 Estimating carbon transfers in the plant rhizosphere. In The Soil-Root Interface. Eds. J LHarley and R SRussel. pp 183–196. Academic Press, London, UK.
Warembourg F R, Montagne D and Bardin R 1982 The simultaneous use of 14CO2 and 15N2 labelling techniques to study the carbon and nitrogen economy of legumes grown under natural conditions. Physiol. Plant. 56, 46–55.
Whipps J M 1984 Environmental factors affecting the loss of carbon from the roots of wheat and barley seedlings. J. Exp. Bot 35, 767–773.
Whipps J M 1990 Carbon economy. In The Rhizosphere. Ed. J MLynch. pp 59–97. Wiley and Sons, Chichester, UK.
Whipps J M and Lynch J M 1983 Substrate flow and utilization in the rhizosphere of cereals. New Phytol. 95, 605–623.
Whipps J M and Lynch J M 1985 Energy losses by the plant in rhizodeposition. Ann. Proc. Phytochem. Soc. Eur. 26, 59–71.
Zadoks J C, Chang T T and Konzak C F 1974 A decimal code for the growth stages of cereals. Weed Res. 14, 415–421.
Author information
Authors and Affiliations
Additional information
Communication No. 73 of the Dutch Programme on Soil Ecology of Arable Farming Systems.
Communication No. 73 of the Dutch Programme on Soil Ecology of Arable Farming Systems.
Rights and permissions
About this article
Cite this article
Swinnen, J. Evaluation of the use of a model rhizodeposition technique to separate root and microbial respiration in soil. Plant Soil 165, 89–101 (1994). https://doi.org/10.1007/BF00009966
Issue Date:
DOI: https://doi.org/10.1007/BF00009966