Abstract
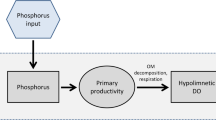
The pH of undisturbed surface waters in the New Jersey Pine Barrens is very acidic (pH 3.5–4.5). Surface waters disturbed by residential and agricultural development typically exhibit significantly elevated pH. Detailed analyses of a disturbed and undisturbed pond showed that the elevated pH of the disturbed pond was primarily the result of enhanced primary productivity. At night and during the winter, when productivity was reduced, the pH dropped to levels more characteristic of the undisturbed pond. The phenomenon of fluctuating pH had significant implications for the zooplankton in the disturbed pond. Though nutrients were significantly greater in the disturbed pond, zooplankton species composition and general abundance patterns were nearly identical in the two ponds. Thus, in these and other poorly buffered waters, it is hypothesized that the lowest pH values were the ones effectively regulating the zooplankton community.
Similar content being viewed by others
References
American Public Health Association, 1980. Standard methods for the examination of water or wastewater. 15th Edn A. P. H. A., Inc., N. Y. 1193 pp.
Brewer, P. G. & J. C. Goldman, 1976. Alkalinity changes generated by phytoplankton growth. Limnol. Oceanogr. 21: 108–117.
Calvayrac, R., J. L. Bomsel & L. M. Danielle, 1979. Analysis and characterization of 3-(3,4-Dichlorophenyl)-1, 1-Dimethylurea (DCMU)-resistant Euglena. Pl. Physiol. 63: 857–865.
Crerar, D. A., J. L. Means, R. F. Yuretich, M. P. Borcsik, J. L. Amster, D. W. Hastings, G. W. Knox, K. E. Lyon & R. F. Quiett, 1981. Hydrogeochemistry of the New Jersey Coastal Plain 2. Transport and deposition of iron, aluminium, dissolved organic matter, and selected trace elements in stream, ground- and estuary water. Chem. Geol. 33: 23–44.
Faust, S., 1983. Total alkalinity versus buffer value (capacity) as a sensitive indicator for fresh waters receiving acid rain. J. envir. Sci. Hlth A18: 701–711.
Forman, R. T. T. (ed.), 1979. Pine Barrens: Ecosystem and landscape. Academic Press, N. Y., 601 pp.
Goldman, J. C. & P. G. Brewer, 1980. Effect of nitrogen source and growth rate on phytoplankton mediated changes in alkalinity. Limnol. Oceanogr. 25: 352–357.
Haines, T. A., 1981. Acidic precipitation and its consequences for aquatic ecosystems: A review. Trans. am. Fish. Soc. 111: 669–707.
Kelley, C. A., J. W. M. Rudd, R. B. Cook & D. W. Schindler, 1982. The potential importance of bacterial processes in regulating rate of lake acidification. Limnol. Oceanogr. 27: 868–882.
Krug, E. C. & C. R. Frink, 1983. Acid rain on acid soils: A new perspective. Science 221: 520–525.
Morgan, M. D., 1984. Acidification of headwater streams in the New Jersey Pinelands: A reevaluation. Limnol. Oceanogr. 29: 1259–1266.
Morgan, M. D., 1983. Impact of changing acidity on the trophic dynamics of Pine Barrens plankton communities. Cent. Cst. Envir. Stud., Rutgers Univ., 38 pp.
National Research Council Canada, 1981. Acidification in the Canadian Aquatic Environment. Natn. Res. Coun. Can, Publ. 18475, 369 pp.
O'Brien, W. J. & F. deNoyelles, Jr. 1972. Photosynthetically elevated pH as a factor in zooplankton mortality in nutrient enriched ponds. Ecology 53: 605–614.
Oliver, G. G. & J. R. M. Kelso, 1983. A role of sediments in retarding the acidification of headwater lakes. Wat. Air Soil Pollut. 20: 379–389.
Pinelands Commission, 1980. New Jersey Pinelands comprehensive management plan. N. Jersey Pinelands Comm., New Lisbon, N.J., 446 pp.
Rhodehamel, E. C., 1979. Hydrology of the New Jersey Pine Barrens. In R. T. T. Forman (ed.), Pine Barrens: Ecosystem and landscape. Academic Press, N. Y.: 147–167.
Schindler, D., R. Wagemann, R. Cook, T. Ruszczynski & J. Prokopowich, 1980. Experimental acidification of Lake 223, Experimental Lakes Area: Background data and the first three years of acidification. Can J. Fish. aquat. Sci. 37: 342–354.
Singer, P. C. & W. Stumm, 1970. Acidic mine drainage: The rate determining step. Science 167: 1121–1123.
Stumm, W. & J. J. Morgan, 1981. Aquatic Chemistry. Wiley-Interscience, N. Y. 780 pp.
Talling, J. E., 1976. The depletion of carbon dioxide from lakewater by photosynthesis. J. Ecol. 64: 79–121.
Tedrow, J. C. F., 1979. Development of Pine Barrens Soils. In R. T. T. Forman (ed.), Pine Barrens: Ecosystem and landscape. Academic Press, N. Y.: 61–79.
Zimmerman, A. P. & H. H. Harvey, 1979. Sensitivity to acidification of waters of Ontario and neighboring states. Final Rep. Ont. Hydro, Univ. Toronto, 118 pp.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Morgan, M.D. Photosynthetically elevated pH in acid waters with high nutrient content and its significance for the zooplankton community. Hydrobiologia 128, 239–247 (1985). https://doi.org/10.1007/BF00006820
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00006820