Abstract
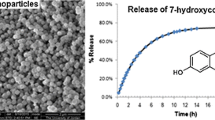
In the presented study, inorganic mesoporous material was used to enhance the solubility of the poorly soluble drug, i.e. dasatinib. The formulation was statistically developed by 33 full factorial design for pharma industry perspective, emphasized by the regulatory authority. The developed formulation was further evaluated for physical stability as per ICH guideline, which is another requirement of the Food and Drug Administration. The effect of critical parameters such as the concentration of drug solution, the stirring rate, and the drug: carrier weight ratio was evaluated statistically and optimized for drug loading to MSU-H nanoparticles. Physical stability of the drug-loaded nanoparticles was performed by accelerated stability protocol given by ICH guideline. Dasatinib loaded MSU-H nanoparticulate system revealed a remarkable dissolution enhancement when compared to the pure crystalline drug and marketed formulation in all tested conditions. Drug-loaded nanoparticles were physically checked within the mesopores of MSU-H nanoparticles and indicated good stability.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
M.V. Regi, Chem. Eur. J. 12(23), 5934–5943 (2006)
J.S. Chang, J.S. Hwang, S.E. Park, ResChem. Intermed. 29(7), 921–938 (2003)
S.M. Van, V. Barillaro, T.D. Thi, R. Mellaerts, J. Martens, J.V. Humbeeck, J. Vermant, P. Annaert, M.G. Vanden, P. Augustijns, J. Pharm. Sci. 98(8), 2648–2658 (2009)
A. Simionesco, C. Coasne, B. Dosseh, G. Dudziak, G. Gubbins, K. Radhakrishnan, R. Sliwinska, M. Bartkowiak, J. Phys. Condens Mat. 18(6), 15–68 (2006)
M. Alcoutlabi, G.B. McKenna, J. Phys. Condens Mat. 17, 461–524 (2005)
J. Vandervoort, A. Ludwig, Pharmazie. 56(6), 484–488 (2001)
N. Vadia, S. Rajput, Eur. J. Pharma. Sci. 45, 8–18 (2012)
G. Box, W. Hunter, J. Hunter, Statistics for Experiments (Wiley, New York, 1978), pp. 291–334
W.G. Cochran, G.M. Cox, Experimental Designs, 2nd edn. (Wiley, New York, 1992), pp. 335–375
U. Ciesla, F. Schüth, Micropor. Mesopor. Mater. 27(2), 131–149 (1999)
G.E. Schulz, J. Rathousky, A. Zukal, Micropor. Mesopor. Mater. 27, 273–285 (1999)
S. Brunauer, P. Emmet, E. Teller, J. Am. Chem. Soc. 60(2), 309–319 (1938)
J. Choma, M. Jaroniec, M.W. Burakiewicz, M. Kloske, Appl. Surf. Sci. 196, 216–223 (2002)
ICH Harmonized Tripartite Guidelines—Stability Testing of New drug Substances and Products Q1A (R2), IFPMA, Geneva (2003)
J. Salonen, L. Laitinen, A.M. Kaukonen, J. Tuura, M. Björkqvist, T. Heikkilä, H.K. Vähä, J. Hirvonen, V.P. Lehto, J. Control. Rel. 108(11), 362–374 (2005)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Vadia, N., Rajput, S. (2019). Statistically Designed Formulation Development of Mesoporous Nanoparticulate Drug Delivery System of Dasatinib for Improved Dissolution and Drug Stability. In: Singh, D., Das, S., Materny, A. (eds) Advances in Spectroscopy: Molecules to Materials. Springer Proceedings in Physics, vol 236. Springer, Singapore. https://doi.org/10.1007/978-981-15-0202-6_20
Download citation
DOI: https://doi.org/10.1007/978-981-15-0202-6_20
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-0201-9
Online ISBN: 978-981-15-0202-6
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)