Abstract
Insoluble radioactive particles have been found in the terrestrial, aquatic and aerial environments. Hot particles are well known as insoluble radioactive particles found after the nuclear tests and the accident at Chernobyl Nuclear Power Plant (CNPP). Hot particles are highly radioactive pieces and mainly composed of nuclear fuel. Insoluble radioactive particles were also found following the Fukushima Daiichi Nuclear Power Plant (FNPP) accident. The particles dissipated from FNPP are almost made of amorphous silica and condensed radioactive cesium (Cs); therefore, they are referred to as radioactive Cs-bearing particles. Radioactive Cs-bearing particles show radioactivity several orders of magnitude lower than hot particles; however, their adverse effects on human health are of great concern. This article summarizes physicochemical properties of radioactive Cs-bearing particles so far reported and discusses their biological effects.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Fukushima Daiichi Nuclear Power Plant accident
- Radioactive cesium
- Insoluble particles
- Biological effects
1 Radioactive Particles
Nuclear tests and nuclear accidents disperse large amounts of radioactive substances into the environment [1, 2]. Following radioactive events, radioactive particles are occasionally found not only in the terrestrial and aerial but also in aquatic environments due to their insoluble nature [3, 4]. As these radioactive particles are derived from nuclear weapons or nuclear fuels, they are generally referred to as “hot particles.” Japanese fishermen were exposed to the fallout from the Castle Bravo nuclear test at Bikini Atoll on March 1, 1954, which was a representative event leading to exposure to hot particles. Hot particles are also generated during normal operations at nuclear installations, and it has been reported that hot particles were inadvertently scattered early in the development of nuclear power [5]. Current concerns center around particles related to nuclear installations. Hot particles of the CNPP accident ranged from hundreds to a few micrometers in size [6]. A study conducted in Poland showed that radioactivity of hot particles from the CNPP accident ranged 1–19 kBq per particle [7]. Radioactive particles were also found after the FNPP accident initially in aerosol filters. Those particles were composed of fission products, mainly radioactive cesium (Cs) as well as radioactive iron (Fe), zinc (Zn) and fuel uranium (U) [8, 9]. Iron was used in FNPP and Zn was added to the primary cooling water for regulation to reduce cobalt-60 (60Co). These observations indicate that FNPP particles were formed in the nuclear reactors. FNPP particles were also found in the terrestrial environment, and their composition was similar to the particles found in the aerosol filters. FNPP particles consist primarily of highly oxidized silicate (Si) glass (a particle contains approximately 70 wt. % of SiO2). The compositions of FNPP particles are markedly different from those in the debris [10] and radioactive Cs was condensed in almost all representative particles [11]. Details of physicochemical characteristics or classification are described in Chapter 15 of this book by Ninomiya. FNPP particles have been reported under different names and are referred to as radioactive Cs-bearing particles in this article. Radioactive Cs-bearing particles are classified into type A and B. Type A particles are typically spherical and a few micrometer in size. Type B particles are larger in size, ranging tens to hundreds of microns and are irregular but not fibrous in shape.
2 Movement of Insoluble Particles in the Respiratory Tract
Since the respiratory tract is in contact with the air through the breathing, radioactive Cs-bearing particles in the air could be inhaled. Therefore, understanding the motion of particles in the respiratory tract is fundamental to evaluate biological effects by inhaled particles. Particle size is a defining factor in prediction of deposition site in the respiratory system. The particles with greater momentum are likely to deposit at the site where the direction of bulk airflow changes rapidly and the larger particles mostly deposit at the bend and bifurcation in upper airways. Several models exist to predict particle motion in the respiratory tract. The International Commission on Radiological Protection (ICRP) Task Group on lung dynamics published a lung model for estimating deposition in and clearance from the respiratory tract and revised it as publication 66 in 1994 [12]. The National Council on Radiation Protection and Measurements (NCRP) independently proposed a respiratory tract dosimetry model using different empirical deposition equations from the ICRP model [13]. Yeh et al. simulated particle motion in naso-oro-pharyngolaryngeal (NOPL), tracheobronchial and pulmonary regions using both ICRP and NCRP models and compared the deposition fraction to the particle size [14]. Both models predict a similar distribution of deposition fraction in tracheobronchial and pulmonary regions for particles of 1–10 μm in size, which are type A particles. Both models also predict a similar distribution of particles between 1 and 2.5 μm in NOPL. However, the ICRP model anticipates that the deposition fraction of 2.5–10 μm particles in the NOPL region is higher than the deposition fraction predicted by the NCRP model.
Based on simulation using the ICRP model, type A particles are capable of entering the deep lung. Type A particles preferentially deposit in the NOPL region, and the particles that pass through the NOPL region subsequently deposit in the tracheobronchial and the pulmonary regions. In contrast, particles of larger than 10 μm, that is, type B particles are preferentially deposited by the settling and impaction mechanism in the NOPL region and at bends in the airflow, including the first few generations of bronchi. Most type B particles do not reach the alveoli [15].
Interaction of particles with tissues of the respiratory tract may result in deleterious effects on human health. Following deposition, it is crucial to remove these particles by physiological clearance processes. Large insoluble particles deposited in the NPOL region are predominantly removed by mucociliary clearance. In this mechanism, the mucus layer traps particles and cilia remove particles from the airways by the coordinated beating. The mucus layer also covers the surface of conducting airways to terminal bronchioles, and the insoluble particles deposited in these regions are also eliminated by mucociliary clearance. The velocity of cilia transport slows down as the particles move further into airways, and mucus transport rate is considered to be 100–600 μm/min in the terminal bronchiole and 5–20 mm/min in the trachea.
Mucociliary clearance does not occur in the gas exchange region. In this region insoluble particles are phagocytosed by alveolar macrophages and are eliminated by the mucociliary escalator [16]. Macrophage clearance depends on particle size and shape. Alveolar macrophages are less efficient at phagocytosing ultrafine particles smaller than 0.1 μm in size or fibrous particles [17,18,19,20]. Ultrafine particles retained in the gas exchange region may be translocated to the lung epithelial lining by endocytosis and by caveolae vesicles [21]. It is currently unknown if irregular shape characteristic of type B particles interferes with phagocytosis by alveolar macrophage.
Radioactive Cs-bearing particles primarily consist of SiO2 at approximately 70 wt.% and are amorphous [22]. High occupational exposure to Si particles leads to silicosis, and internalization of amorphous Si particles into macrophages has been studied. Size-specific cellular uptake of amorphous Si particles was evaluated using macrophages experimentally differentiated from THP-1, a human acute monocytic leukemia cell line. Macrophages incorporated ultrafine particles more effectively than micronparticles. Class A scavenging receptors such as SR-A1 and SR-A6 are involved in cellular uptake of amorphous Si particles, and both receptors exhibit size-specific cellular uptake of particles with approximately 50 and 100 nm, but not 1 μm in diameter, indicating that macrophages recognize radioactive Cs-bearing particles via SR-As-independent mechanisms [23, 24]. Recently, SR-B1 was identified as a Si receptor and recognized various sizes of amorphous Si, including the particles with 1 μm in size [25]. However, SR-B1 recognizes and tethers the particles on the cell surface, but internalization into rodent lymphoma cells or fibroblasts expressing mSR-B1 did not occur. The fate of macrophage incorporating or tethering radioactive Cs-bearing particles is unknown and should be examined further.
3 Perspective on Biological Impact of Exposure to Radioactive Cs-Bearing Particles
The biological impact of exposure to radioactive Cs-bearing particles have become a prominent concern since discovery of these particles. Controversy relating to the biological impact of insoluble radioactive particles began in the late 1960s in connection with lung exposure to hot particles. Hot particles are physically small but cause relatively high local dose of radiation and ICRP discussed carcinogenesis in the lung exposed to hot particles.
3.1 Fate and the Effect of Type B Particles
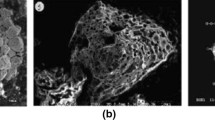
Our preliminary experiments demonstrated that type B particles have a potential to affect surrounding cells. Human retinal pigmented epithelial cells immortalized by telomerase transfection were seeded at lower density (upper images in Fig. 16.1), and cell proliferation was monitored by live-cell imaging up to 53 h from the beginning of co-culture with a type B particle with more than 1000 Bq of 137Cs. This 53-h period of co-culture is sufficient for the cell culture to become confluent without radioactive Cs-bearing particles. Cell density in the region not directly contacting the particle increased by the end of live-cell imaging, while the region in direct contact with the particle was sparse (bottom images in Fig. 16.1). The shape of the cells near the particle was larger and flatter than proliferating cells. These morphological changes are representative of cellular senescence, as persistent cell cycle arrest is one of the features of senescent cells. Since ionizing radiation induces cellular senescence, senescence-like phenotype may be elicited in the cells directly attached to the radioactive Cs-bearing particle. We extracted total RNA from three groups of cells; co-cultured with a type B particle, co-cultured with a nonradioactive mock particle (mock particle) and without a particle, respectively, and gene expression level for 21,088 genes was investigated by microarray analysis (3D-Gene Human Oligo chip 25k, TORAY, Kanagawa, Japan). As a control study, a mock particle composed of SiO2 and stable cesium was generated. Expression of 1,944 genes was at least 1.5 fold different between cells co-cultured with a mock particle and those without, indicating that the physical presence of the particle influences gene expression. In total, 2,455 genes showed at least 1.5 fold different between cells treated with a radioactive Cs-bearing particle and the mock control. Type B particle increased the expression of cell cycle suppressors such as CDKN1C, CDKN1B and GADD45A, and reduced the expression of cell cycle promoters such as cyclin E2, cyclin B1 and cyclin A2. These results demonstrated that type B particles suppress cell growth, consistent with our observation using live-cell imaging. Ionizing radiation triggers activation of cell cycle checkpoints, resulting in cell cycle arrest in the G1 and G2/M phases. However, cell cycle suppressor genes upregulated by radioactive Cs-bearing particle are not involved in checkpoint regulation. Therefore, further studies are needed to elucidate how radioactive Cs-bearing particles suppress cell growth.
Live-cell imaging of normal human epithelial cells
Cells not in contact with the particle (A) and those in contiguity with the particle (B) were monitored by a live-cell imaging. The images were taken at the beginning (upper) and the end (lower) of the culture. A type B particle is seen at the left side of images (B)
3.2 The Effect of Type A Particles
Due to the larger size of type B particles, inhaled type B particles are predicted to be removed by mucociliary clearance without entering into the deep lung region. In contrast, type A particles could enter the alveolar region. Macrophages function to recognize, take up and clear Si particles, but macrophages may fail to remove insoluble Si particles from the lung. Internalization of insoluble particles into macrophages triggers lysosomal stress, resulting in cell death pathway [26, 27]. In addition, macrophages tether Si particles on their cellular surface, but do not internalize when SR-B1 mediates recognition of Si particles [25]. The fate of the macrophage tethering insoluble particles is not clear, but the signaling pathway associated with lysosomal stress is activated in particle-bound macrophages. Regardless of whether macrophages take up or tether radioactive Cs-bearing particles, these particles may ultimately be retained in the alveolar region, resulting in the increased risk of lung injury. Furthermore, radioactive Cs-bearing particles can become a source of chronic radiation exposure to surrounding cells. Since alveolar macrophages are known to be radioresistant [28], other cells present in alveoli such as fibroblasts, epithelial cells and endothelial cells may be the most susceptible to chronic radiation exposure. When analyzing biological effects of radioactive Cs-bearing particles, bystander effects should be considered.
Radioactive Cs-bearing particles emit β-particles and γ-rays, and β-particles dissipate within a short distance. Therefore, spatial distributions of radiation doses around these particles are nonuniform and bystander effect may not be ignored. Several reports suggest that radioactive Cs-bearing particles may physically trigger cellular reactions, which may result in additive or synergistic effects for low-dose (LD) radiation and inflammatory responses [29,30,31]. Radiation-induced bystander effect is characterized by the appearance of radiation effects in non-irradiated cells adjacent to exposed cells. Bystander effect is mediated by either soluble factors secreted from irradiated cells [32,33,34] or gap junction communication which allows molecular signals to pass through protein channels between adjacent cells [35, 36]. Bystander factors are associated with various effects in adjacent non-irradiated cells such as induction of DNA damages, chromosomal aberrations, gene mutations and cell death, and alterations in epigenetic status including gene expression and cell cycle regulation [37,38,39]. Although a unified mechanism has not been discovered, it is assumed that molecules relating to DNA damage response such as ATM and p53 are involved in the expression of bystander effect [36, 40, 41]. Bystander effect occurs both in vivo and in vitro [42, 43]. Aside from the canonical bystander effect, a pathway from non-irradiated bystander cells to the primarily irradiated cells has been reported. For example, DNA damage in irradiated cells is mitigated by co-culture with non-irradiated bystander cells [44]. High-dose single radiation exposure induces cellular senescence in somatic cells, which is referred to stress-induced premature senescence (SIPS) [45]. LD fractionated irradiation (5 cGy) or chronic low-dose-rate (LDR) (4.1 mGy/h) radiation exposure also elicits SIPS [46, 47]. Cells undergoing SIPS secrete proteins related to inflammatory responses or radiation resistance enhancement [48, 49]. Since the SIPS phenotype is irreversible, prolonged inflammatory microenvironment induced by radioactive Cs-bearing particles has potential to contribute to lung fibrosis.
4 Conclusion
This article reviewed potentially hazardous biological effects of radioactive Cs-bearing particles produced by the FNPP accident. Our preliminary in vitro data demonstrated that a type B particle interrupts the growth of adjacent cells. Additive or synergistic biological effects of Si component should be considered when studying the health effect of persistent LDR radiation by the particles. Animal experiments using radioactive Cs-bearing particles are necessary to investigate the distribution of ingested particles and their tissue effects, including cancer induction.
References
Crocker GR, O’Connor JD, Freiling EC (1966) Physical and radiochemical properties of fallout particles. Health Phys 12(8):1099–1104
Schmidt-Burbach GM (1970) Experimental determination of the distribution of dose rate around punctiform radioactive particles. Health Phys 18(3):295–296
Smith JN, Ellis KM, Aarkrog A et al (1994) Sediment mixing and burial of the 239,240Pu pulse from the 1968 Thule, Greenland nuclear weapons accident. J Environ Radioact 25:135–159
Cutshall N, Osterberg C (1964) Radioactive particle in sediment from the Columbia River. Science 144(3618):536–537
Salbu B, Bjørnstad HE, Svarenb HE et al (1993) Size distribution of radionuclides in nuclear fuel reprocessing liquids after mixing with seawater. Sci Total Environ 130/131:51–63
Sandalls FJ, Segal MG, Victorova N (1993) Hot particles from Chernobyl: a review. J Environ Radioact 18:5–22
Osuch S, Dabrowska M, Jaracz P et al (1989) Isotopic composition of high-activity particles released in the Chernobyl accident. Health Phys 57(5):707–716
Adachi K, Kajino M, Zaizen Y et al (2013) Emission of spherical cesium-bearing particles from an early stage of the Fukushima nuclear accident. Sci Rep 3:2554
Abe Y, Iizawa Y, Terada Y et al (2014) Detection of uranium and chemical state analysis of individual radioactive microparticles emitted from the Fukushima nuclear accident using multiple synchrotron radiation X-ray analyses. Anal Chem 86(17):8521–8852
Burns PC, Ewing RC, Navrotsky A (2012) Nuclear fuel in a reactor accident. Science 335(6073):1184–1188
Satou Y, Sueki K, Sasa K et al (2016) First successful isolation of radioactive particles from soil near the Fukushima Daiichi Nuclear Power Plant. Anthropocene 14:71–77
International Commission on Radiological Protection (ICRP) (1994) Human respiratory tract model for radiological protection. Ann ICRP 24(1–3). ICRP publication 66
National Council of Radiation Protection and Measurements (NCRP) (1997) Deposition, retention, and dosimetry of inhaled radioactive substances. NCRP report no. 125
Yeh H-C, Cuddihy RG, Phalen RF, I-Y C (1996) Comparisons of calculated respiratory tract deposition of particles based on the proposed NCRP model and the new ICRP66 model. Aerosol Sci Technol 25:134–140
Zhang Z, Kleinstreuer C, Donohue JF et al (2005) Comparison of micro- and nano-size particle depositions in a human upper airway model. J Aerosol Sci 36(2):211–233
Lay JC, Bennett WD, Kim CS et al (1998) Retention and intracellular distribution of instilled iron oxide particles in human alveolar macrophages. Am J Respir Cell Mol Biol 18:687–695
Oberdörster G (1993) Lung dosimetry: pulmonary clearance of inhaled particles. Aerosol Sci Technol 18(3):279–289
Kreyling WG, Semmler-Behnke M, Moller W (2006) Ultrafine particle-lung interactions: does size matter? J Aerosol Med 19(1):74–83
Karakoti AS, Hench LL, Seal S (2006) The potential toxicity of nanomaterials—the role of surfaces. JOM 58(7):77–82
Stanton MF, Layard M, Tegeris A et al (1977) Carcinogenicity of fibrous glass: pleural response in the rat in relation to fiber dimension. J Natl Cancer Inst 58(3):587–603
Oberdörster G, Oberdörster E, Oberdörster J (2005) Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 113(7):823–839
Satou Y, Sueki K, Sasa K et al (2018) Analysis of two forms of radioactive particles emitted during the early stages of the Fukushima Daiichi Nuclear Power Station accident. Geochem J 52(2):137–143
Hamilton RF Jr, Thakur SA, Mayfair JK et al (2006) MARCO mediates silica uptake and toxicity in alveolar macrophages from C57BL/6 mice. J Biol Chem 281(45):34218–34226
Orr GA, Chrisler WB, Cassens KJ et al (2011) Cellular recognition and trafficking of amorphous silica nanoparticles by macrophage scavenger receptor A. Nanotoxicology 5(3):296–311
Tsugita M, Morimoto N, Tashiro M et al (2017) SR-B1 is a silica receptor that mediates canonical inflammasome activation. Cell Rep 18(5):1298–1311
Costantini LM, Gilberti RM, Knecht DA (2011) The phagocytosis and toxicity of amorphous silica. PLoS One 6(2):e14647
Thibodeau MS, Giardina C, Knecht DA et al (2004) Silica-induced apoptosis in mouse alveolar macrophages is initiated by lysosomal enzyme activity. Toxicol Sci 80(1):34–48
Oghiso Y, Yamada Y (1992) Heterogeneity of the radiosensitivity and origins of tissue macrophage colony-forming cells. J Radiat Res 33:334–341
Huh D, Matthews BD, Mammoto A et al (2010) Reconstituting organ-level lung functions on a chip. Science 328:1662–1668
Kusaka T, Nakayama M, Nakamura K et al (2014) Effect of silica particle size on macrophage inflammatory responses. PLoS One 9(3):e92634
Merget R, Bauer T, Küpper H et al (2001) Health hazards due to the inhalation of amorphous silica. Arch Toxicol 75(11–12):625–634
Lehnert BE, Goodwin EH (1997) Extracellular factor(s) following exposure to a particles can cause sister chromatid exchanges in normal human cells. Cancer Res 57:2164–2171
Mothersill C, Seymour CB (1998) Cell-cell contact during gamma irradiation is not required to induce a bystander effect in normal human keratinocytes: evidence for release during irradiation of a signal controlling survival into the medium. Radiat Res 149:256–262
Sowa Resat MB, Morgan WF (2004) Radiation-induced genomic instability: a role for secreted soluble factors in communicating the radiation response to non-irradiated cells. J Cell Biochem 92(5):1013–1019
Mancuso M, Pasquali E, Leonardi S et al (2008) Oncogenic bystander radiation effects in patched heterozygous mouse cerebellum. Proc Natl Acad Sci U S A 105(34):12445–12450
Azzam EI (2001) Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha-particle irradiated to nonirradiated cells. Proc Natl Acad Sci U S A 98(2):473–478
Nagasawa H, Little JB (2002) Bystander effect for chromosomal aberrations induced in wild-type and repair deficient CHO cells by low fluences of alpha particles. Mutat Res 508:121–129
Persaud R, Zhou H, Baker SE et al (2005) Assessment of low linear energy transfer radiation–induced bystander mutagenesis in a three-dimensional culture model. Cancer Res 65(21):9876–9882
Lyng FM, Seymour CB, Mothersill C (2002) Initiation of apoptosis in cells exposed to medium from the progeny of irradiated cells: a possible mechanism for bystander-induced genomic instability? Radiat Res 157:365–370
Hagelstrom RT, Askin KF, Williams AJ et al (2008) DNA-PKcs and ATM influence generation of ionizing radiation-induced bystander signals. Oncogene 27(53):6761–6769
Azzam EI, de Toledo SM, Gooding T et al (1998) Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of alpha particle. Radiat Res 150:497–504
Hatzi VI, Laskaratou DA, Mavragani IV et al (2015) Non-targeted radiation effects in vivo: a critical glance of the future in radiobiology. Cancer Lett 356(1):34–42
Chai Y, Lam RK, Calaf GM et al (2013) Radiation-induced non-targeted response in vivo: role of the TGFbeta-TGFBR1-COX-2 signalling pathway. Br J Cancer 108(5):1106–1112
He M, Dong C, Xie Y et al (2014) Reciprocal bystander effect between alpha-irradiated macrophage and hepatocyte is mediated by cAMP through a membrane signaling pathway. Mutat Res 763–764:1–9
Suzuki M, Boothman DA (2008) Stress-induced premature senescence (SIPS). J Radiat Res 49(2):105–112
Tsai KKC, Stuart J, Y-YE C et al (2009) Low-dose radiation-induced senescent stromal fibroblasts render nearby breast cancer cells radioresistant. Radiat Res 172(3):306–313
Rombouts C, Aerts A, Quintens R et al (2014) Transcriptomic profiling suggests a role for IGFBP5 in premature senescence of endothelial cells after chronic low dose rate irradiation. Int J Radiat Biol 90(7):560–574
Aggarwal BB, Gehlot P (2009) Inflammation and cancer: how friendly is the relationship for cancer patients? Curr Opin Pharmacol 9(4):351–369
Klokov D, Leskov K, Araki S et al (2013) Low dose IR-induced IGF-1-sCLU expression: a p53-repressed expression cascade that interferes with TGFbeta1 signaling to confer a pro-survival bystander effect. Oncogene 32(4):479–490
Acknowledgment
This work was supported by the grant provided by the Nuclear Energy Science & Technology and Human Resource Development Project (through Concentrating Wisdom) #281302 to MS and JSPS KAKENHI Grant #26253022 to MF from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2020 The Author(s)
About this chapter
Cite this chapter
Suzuki, M., Ninomiya, K., Satou, Y., Sueki, K., Fukumoto, M. (2020). Perspective on the Biological Impact of Exposure to Radioactive Cesium-Bearing Insoluble Particles. In: Fukumoto, M. (eds) Low-Dose Radiation Effects on Animals and Ecosystems. Springer, Singapore. https://doi.org/10.1007/978-981-13-8218-5_16
Download citation
DOI: https://doi.org/10.1007/978-981-13-8218-5_16
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-8217-8
Online ISBN: 978-981-13-8218-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)