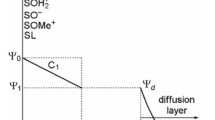
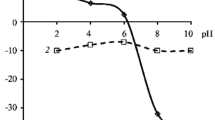
Abstract
This chapter is concerned with some aspects of the interaction of oxide/hydroxide surfaces with electrolyte solutions. It specially covers interactions with hydrogen- and hydroxide ions, with metal ions (Lewis to the (hydr)oxide-water interface. It leads to an accumulation of matter at the interface without the development of a three dimensional molecular structure (Sposito, 1986). This step is usually considered to be fast and reversible. It is followed by a series of slow and at least partially irreversible processes (Figure 4.1). The nature of these processes is dependent on the prevailing surface coverage. Adsorption of charged species results in a change of surface charge and surface potential (see Chapter 2) and thus in a change in the stability of the colloidal system. Adsorption of hydrogen- and hydroxide ions may induce dissolution of the adsorbing solid (hydr)oxide upon formation of aqua ions or hydroxo complexes (Furrer and Stumm, 1986; Pulfer et al., 1984). The rate of dissolution under both acidic and alkaline conditions is often greatly enhanced by the presence of adsorbing anions. Adsorption of metal ions exceeding a critical surface coverage leads to the formation of hydroxide clusters of the adsorbed metal on the adsorbing surface (Bleam and McBride, 1985), a process that has also been termed ‘surface precipitation’ (Farley et al., 1985). For example, simultaneous adsorption of both Ca2+ and F− on CeO2 has been found to be prerequisite for heteronucleation and subsequent growth of CaF2 crystals from the adsorbing CeO2 surface (Hohl et al., 1985). Also, heterogeneous redox reactions (such as corrosion, reductive dissolution of transition metal oxides and processes for conversion of solar energy) are based on adsorption followed by (photochemically assisted) electron transfer.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Baes, C.F. and Mesmer, R.E. 1976. The Hydrolysis of Cations. Wiley, New York.
Bilinski, H. and Ingri, N. 1967. A determination of the formation constant of SiO(OH)3.Acta Chem. Scand. 21, 2503–2510.
Basak, M., Bourg, A.C.M., Cornell, R.M., Gisler, A., Schindler, P.W., Stettler, E. and Trusch, B. 1987. The effect of dissolved ligands upon the adsorption of metal ions at oxide-water interfaces. (to be published. )
Bleam, W.F. and McBride, M.B. 1985. Cluster formation versus isolated-side adsorption. A study of Mn(II) and Mg(II) adsorption on boehmite and goethite. J. Colloid Interface Sci. 103, 124–132.
Bolt, G.H. and Van Riemsdijk, W.H. 1982. Ion adsorption on inorganic variable charge constituents. In G.H. Bolt (ed.), Soil Chemistry. Part B. 2nd edition, Elsevier, Amsterdam. pp. 459–504.
Bourg, A.C.M. and Schindler, P.W. 1978. Ternary surface complexes. I. Complex formation in the system silica-Cu(II)-ethylenediamine. Chimia 32, 166–168.
Davis, JA., James, R.O. and Leckie, J.O. 1978. Surface Ionization and complexation at the oxide/water interface. I. Computation of electrical double layer properties in simple electrolytes. J. Colloid Interface Sci. 63, 480–499.
Davis, JA. and Leckie, J.O. 1978. Effect of adsorbed complexing ligands on trace metal uptake by hydrous oxides. Environ. Sci. Technol. 2, 1309–1315.
Farley, K.J., Dzombak, DA. and Morel, F.M.M. 1985. A surface precipitation model for the sorption of cations on metal oxides. J. Colloid Interface Sci. 106, 226–242.
Fürst, B. 1976. Das koordinationschemische Adsorptionsmodell: Oberflächenkomplexbildung von Cu(II), Cd(II) und Pb(II) an Si02 (Aerosil) und TiO2 (Rutil). Ph.D. Thesis, University of Bern, Bern, Switzerland.
Furrer, G. and Stumm, W. 1980. The coordination chemistry of weathering. I. Dissolution kinetics of S-Al203 and BeO. Geochim. Cosmochim. Acta 50, 1847–1860.
Gisler, A. 1980. Die Adsorption von Aminosäuren an Grenzflächen Oxid-Wasser. Ph.D. Thesis, University of Bern, Bern, Switzerland.
Goldberg, S. and Sposito, G. 1984a. A chemical model for phosphate adsorption by soils. I. Reference oxide minerals. Soil. Sci. Soc. Amer. J. 48, 772–778.
Goldberg, S. and Sposito, G. 1984b. A chemical model for phosphate adsorption by soils. II. Noncalcareous soils. Soil. Sci. Soc. Amer. J. 48, 779–783.
Grauer, R. and Stumm, W. 1982. Die Koordinationschemie oxidischer Grenzflächen und ihre Auswirkung auf die Auflösungskinetik oxidischer Festphasen in wässerigen Lösungen. Colloid & Polymer Sci. 260, 959–970.
Hachiya, K., Sasaki, M., Ideka, T., Mikami, N., and Yasunaga, T. 1984. Static and kinetic studies of adsorption-desorption of metal ions on ry-Al203 surface. 2. Kinetic study by means of pressure jump technique. J. Phys. Chem. 88, 27–31.
Hohl, H. and Stumm, W. 1976. Interaction of Pb2+ with hydrous y-Al203. J. Colloid Interface Sci. 55, 281–288.
Hohl, H., Werth, E., Giovanoli, R. and Posch, E. 1985. Heterogeneous Nucleation. I. Nucleation of calcium fluoride on cerium (IV) oxide. Unpublished report as quoted in P.W. Schindler (1985).
Huang, C.P. and Stumm, W. 1973. Specific adsorption of cations on hydrous -Al2O3.y J.
Colloid Interface Sci 43 409–420.
Huang, C.P. 1981. The Surface Acidity of Hydrous Solids. In M.A. Anderson and A.J. Rubin (eds.), Adsorption of Inorganics at Solid-Liquid Interfaces. Ann Arbor Science, Ann Arbor, Michigan. pp. 183–217.
James, R.O. and Healy, T.W. 1972. Adsorption of hydrolyzable metal ions at the oxide-water interface. I, II and III. J. Colloid Interface Sci. 40, 42–81.
Johnson, DA. 1982. Some Thermodynamic Aspects of Inorganic Chemistry Cambridge University Press.
Kawakami, H. and Yoshida, S. 1985. Quantum chemical studies of alumina. I. Bronsted acidity and basicity. J. Chem. Soc. Faraday Trans. 2–81, 1117–1127.
Kiselev, A.V. 1971. The effect of the geometrical structure and the chemistry of oxide surfaces on their adsorption properties. Discuss. Faraday Soc. 52, 14–32.
Koch, S. 1965. Kinetik und Mechanismus der Auflösung von Berylliumoxid in Säuren. Ber. Bunsenges. physik. Chemie 69, 141–145.
Kummert, R. and Stumm, W. 1980. Surface complexation of organic acids on hydrous -y-Al203. J. Colloid Interface Sci. 75, 373–385.
Lagerström, G. 1959. Equilibrium studies of polyanions. III. Silicate ions in NaC1O4. Acta Chem. Scand. 13, 722–736.
Morel, F.M.M., Westall, J.C. and Yeasted, J.G. 1981. Adsorption Models. A Mathematical Analysis in the Framework of General Equilibrium Calculations. In M.A. Anderson and A.J. Rubin (eds.),Adsorption of Inorganics at Solid-Solution Interfaces. Ann Arbor Science, Ann Arbor, Michigan. pp. 263–294.
Motschi, H. 1987. Aspects of the Molecular Structure in Surface Complexes: Spectroscopic Investigations. In W. Stumm (ed.), Aqueous Surface Chemistry. Wiley, New York. pp. 111–125.
Olson, L.L. and O’Melia C.R. 1973. The interactions of Fe(III) with Si(OH)4. J. Inorg. Nucl. Chem. 35, 1977–1985.
Peri, J.B. 1965. A model for the surface of 1-alumina. J. Phys. Chem. 69, 220–230.
Pulfer, K., Schindler, P.W., Westall, J.C. and Grauer, R. 1984. Kinetics and mechanism of
dissolution of bayerite (-y-Al(OH)3) in HNO3-HF solutions at 298.2 K. J. Colloid InterfaceSci. 101,554–564.
Santschi, P. and Schindler, P.W. 1974. Complex formation in the ternary systems
Ca(II)-H4SiO4–H20 and Mg(II)-H4SiO4–H20. J. Chem. Soc. Dalton 2, 181–184.
Schindler, P.W. and Kamber, H.R. 1968. Die Acidität von Silanolgruppen. Hely. Chin. Acta 51, 1781–1786.
Schindler, P.W. and Gamsjäger, H. 1972. Acid-base reactions at the TiO2 (anatase)-water interface and the point of zero charge of TiO2 suspensions. Kolloid-Z. u. Z. Polymere 250, 759–763.
Schindler, P.W., Fürst, B., Dick, R. and Wolf, P.U. 1976. Ligand properties of surface silanol groups. I. Surface complex formation with Fei+, Cu2+, Cd2+ and Pb2+. J. Colloid Interface Sci. 55, 469–475.
Schindler, P.W., Wälti, E. and Fürst, B. 1976. The role of surface hydroxyl groups in the surface chemistry of metal oxides. Chimia 30, 107–109.
Schindler, P.W. 1981. Surface Complexes at Oxide-Water Interfaces. In MA. Anderson and A.J. Rubin (eds.), Adsorption of Inorganics at Solid-Liquid Interfaces. Ann Arbor Science, Ann Arbor, Michigan. pp. 1–47.
Schindler, P.W. 1985. Grenzflächenchemie oxidischer Mineralien. Oester. Chem. Z. 86, 141–147.
Schindler, P.W. and Stumm, W. 1987. The Surface Chemistry of Oxides, Hydroxides and Oxide
Minerals. In W. Stumm (ed.), Aquatic Surface Chemistry. Wiley, New York. pp. 83–110.
Sigg, L. 1973. Untersuchungen über Protolyse und Komplexbildung von zweiwertigen Kationen mit Silikageloberflächen. M.Sc. thesis, University of Bern, Bern, Switzerland.
Sposito, G. 1983. On the surface complexation model of the oxide-aqueous solution interface. J. Colloid Interface Sci. 91, 329–340.
Sposito, G. 1984. The Surface Chemistry of Soils. Oxford University Press, New York.
Sposito, G. 1986. Distinguishing adsorption from surface precipitation. In J.A. Davis and K.F.
Hayes (eds.), Geochemical Processes of Mineral Surfaces. ACS Symposium Series No. 323. Am. Chem. Soc., Washington, D.C. pp. 217–228.
Sposito, G. and Schindler, P.W. 1986. Reactions at the Soil Colloid-Soil Solution Interface. Proc. of the XIII. Congress of the International Society of Soil Science VI, 683–699.
Stumm, W., Huang, C.P. and Jenkins, S.R. 1970. Specific chemical interaction affecting the stability of dispersed systems. Croat. Chem. Acta 42, 223–245.
Stumm, W., Kummert, R. and Sigg, L. 1980. A ligand exchange model for the adsorption of inorganic and organic ligands at hydrous oxide interfaces. Croat. Chem. Acta 53, 291–312.
Tamura, H., Matijevic, E. and Meites, L. 1983. Adsorption of Co2+ ions on spherical magnetite particles. J. Colloid Interface Sci. 92, 303–314.
von Zelewsky, A. and Bemtgen, M. 1982. Formation of ternary copper(II) complexes at the surface of silica gel as studied by esr spectroscopy. Inorg. Chem. 21, 1771–1777.
Weber, W.J. Jr. and Stumm, W. 1965. Formation of a silicato-iron(III) complex in dilute aqueous solution. J. Inorg. Nucl. Chem. 27, 237–239.
Westall, J.C. and Hohl, H. 1980. A comparison of electrostatic models for the oxide solution interface. Adv. Colloid Interface Sci. 12, 265–294.
Zabin, B.A. and Taube, H. 1965. The reactions of metal oxides with aquated chromium(II) ion. Inorg. Chem. 3, 963–968.
Zinder, B., Furrer, G. and Stumm, W. 1986. The coordination chemistry of weathering. II. Dissolution of Fe(III)oxides. Geochirn. Cosmochim. Acta 50, 1861–1869.
Bear, F.E. 1964. Chemistry of the Soil. Reinhold, New York, 337pp.
Bohn, H. L., McNeal, B.L. and O’Connor G.A. 1979. Soil Chemistry. Wiley Interscience, New York, 329 pp.
Bolt, G.H. and Bruggenwert, M.G.M. (eds.) 1976. Soil Chemistry. A. Basic Elements. Elsevier, Amsterdam, 281 pp.
Marshall, C.E. 1964. The Physical Chemistry and Mineralogy of Soils. I. Soil Materials. Wiley and Sons, Inc., New York, 387 pp.
Russell, E.W. 1961, 1973. Soil Conditions and Plant Growth. 10th Edition. Longman, London, 849 pp.
Scheffer, F. and Schachtschabel, P. 1970. Lehrbuch der Bodenkunde. 10th Edition. Enke Verlag, Stuttgart, 394 pp.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1991 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Schindler, P.W., Sposito, G. (1991). Surface Complexation at (Hydr)Oxide Surfaces. In: Bolt, G.H., De Boodt, M.F., Hayes, M.H.B., McBride, M.B., De Strooper, E.B.A. (eds) Interactions at the Soil Colloid — Soil Solution Interface. NATO ASI Series, vol 190. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-1909-4_4
Download citation
DOI: https://doi.org/10.1007/978-94-017-1909-4_4
Publisher Name: Springer, Dordrecht
Print ISBN: 978-90-481-4081-7
Online ISBN: 978-94-017-1909-4
eBook Packages: Springer Book Archive