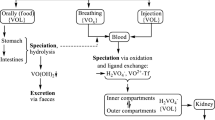
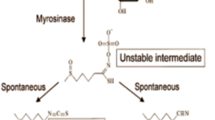
Abstract
The pharmacological role of vanadium in health and disease remains one of the fascinating stories in biology. Recent studies have established vanadium as a novel regulator in assessing physiological and biochemical states of the animals. Vanadium exhibits biphasic effect, essentiality at low concentrations (0.05 μM) and toxicity at high doses (>10 μM). Vanadium inhibits growth of transformed cancer cells in culture. Various laboratories have confirmed the antitumorigenic potential of vanadium in liver, breast and colon cancer in vivo and various human cancer epithelial cell lines in vitro. Antiproliferative and induction of apoptosis may be the major mechanism of vanadium mediated inhibitions of cancer. Vanadium can play a central role in modulating phosphorylation states of various proteins in the cell and can affect many cellular processes regulated by cyclic AMP. In human vanadium is of interest pharmacologically but confirmation to its essentiality will require more significant information from experimental, clinical and epidemiological studies.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Paget S (1889) The distribution of secondary growths in cancer of the breast. Lancet 1: 571–573
Mukherjee B, Patra B, Mahapatra S et al (2004) Vanadium-an element of atypical biological significance. Toxicol Lett 150:135–143
Almedeida M, Filipe S, Hunianes M et al (2001) Vanadium haloperoxidases from brown algae of the Laminariaceae family. Photochemistry 57:633–642
French RJ, Jones PJ (1993) Role of vanadium in nutrition: metabolism, essentiality and dietary consideration. Life Sci 52:339–346
Domingo JL (1996) Vanadium: a review of the reproductive and developmental toxicology. Reprod Toxicol 10:175–182
Anke M, Groppel B, Kosla T et al (1986) New research on vanadium deficiency in ruminants. In: Anke M et al (eds) Supplement-symposium: new trace elements. Friedrich-Schiller-Universitat, Jena, pp 1266–1275
Menon AS, Rau M, Ramasarma T et al (1980) Vanadate inhibit mevalonate synthesis and activates NADH oxidation in microsomes. FEBS Lett 114:139–141
Golden MH, Golden BE (1981) Trace elements. Potential importance in human nutrition with particular reference to zinc and vanadium. Br Med Bull 37:31–36
Barceloux DG (1999) Vanadium. J Toxicol Clin Toxicol 37:265–278
Byrne AR, Kosta L (1978) Vanadium in foods and in human body fluids and tissues. Sci Total Environ 10:17–30
Badmaev V, Prakash S, Majeed M (1999) A review of its potential role in the fight against diabetes. J Altern Complement Med 5:273–291
Mongold JJ, Cros GH, Vian L et al (1990) Toxicological aspects of vanadyl sulphate on diabetic rats: effects on vanadium levels and pancreatic B-cell morphology. Pharmacol Toxicol 67:192–198
Philips TD, Nechay BR, Heidelbaugh ND (1983) Vanadium: chemistry and the kidney. Fed Proc 42:2969–2973
Nechay BR (1984) Mechanisms of actions of vanadium. Annu Rev Pharmacol Toxicol 24:501–524
Orvig C, Thompson KH, Battell M et al (1995) Vanadium compounds as insulin mimics. Met Ions Biol Syst 31:575–594
Kieler J, Gromek A, Nissen N (1965) Studies on the antineoplastic effect of vanadium salts. Acta Chir Scand Suppl 343:154–164
Thompson HJ, Chasten ND, Meeker LD (1984) Dietary vanadyl (IV) sulphate inhibits chemically-induced mammary carcinogenesis. Carcinogenesis 5:849–851
Bishayee A, Chatterjee M (1995) Inhibition of altered liver cell foci and persistent nodule growth by vanadium during diethylnitrosamine-induced hepatocarcinogenesis in rats. Anticancer Res 15:455–462
Harding MM, Mokdsi G (2000) Antitumor metallocenes: structure-activity studies and interactions with biomolecules. Curr Med Chem 7:1289–1303
Murthy MS, Rao LN, Kuo LY et al (2000) Antitumor and toxicologic properties of the organometallic anti-cancer agent vanadocene dichloride. Inorg Chem Acta 152:117–124
WHO (1988) Vanadium. Environmental Health Criteria No. 80. Geneva
Baroch EF (1983) Vanadium and vanadium alloys. In: Seidel A (ed) Encyclopedia of chemical technology, vol 23. Wiley, New York, pp 673–687
Rosenbaum JB (1983) Vanadium compounds. In: Seidel A (ed) Encyclopedia of chemical technology, vol 23. Wiley, New York, pp 688–704
Al-Swaidan HM (1993) Determination of vanadium and nickelin oil products from Saudi Arabia by inductively coupled plasma mass spectrometry (ICP/MS). Anal Lett 26:141–146
Crans DC (1998) Chemistry of relevance to vanadium in the environment. In: Nriagu JO (ed) Vanadium in the environment. Part 1: chemistry and biochemistry. Wiley, New York, pp 73–96
Dafnis E, Sabatini S (1994) Biochemistry and pathophysiology of vanadium. Nephron 67:133–143
Crans DC, Bunch RL, Theisen LA (1989) Interaction of race levels of vanadium (IV) and vanadium (V) in biological systems. J Am Chem Soc 111:7597–7607
Erdmann E, Werdan K, Krawietz W et al (1984) Vanadate and its significance in biochemistry and pharmacology. Biochem Pharmacol 33:945–950
Nechay BR, Nanninga LB, Nechay PSE et al (1986) Role of vanadium in biology. Fed Proc 45:123–132
Ueki H, Okuhama R, Gresser MJ et al (1988) Interaction of vanadate with uridine and adenosine monophosphate: formation of ADP and ATP analogues. J Am Chem Soc 110:5869–5874
Wenzel UO, Fouqueray B, Biswas P et al (1995) Activation of mesangial cells by the phosphatase inhibitor vanadate. J Clin Invest 95:1244–1252
Ding M, Gannett PM, Rojanasakul Y et al (1994) One-electron reduction of vanadate by ascorbate and related free radical generation at physiological pH. J Inorg Biochem 55: 101–112
Shi X, Wang P, Jiang H et al (1996) Vanadium (IV) causes 2′-deoxyguanosine hydroxylation and deoxyribonucleic acid damage via free radical reaction. Ann Clin Lab Sci 26:39–49
Imbert V, Peyron JF, Far F et al (1994) Induction of tyrosine phosphorylation and T-cell activation by vanadate peroxide, an inhibitor of protein tyrosine phosphatases. Biochem J 297:163–173
Yamaguchi M, Oishi H, Araki S et al (1995) Respiratory burst and tyrosine phosphorylation by vanadate. Arch Biochem Biophys 323:382–386
Kalyani P, Vijaya S, Ramasarma T (1992) Characterisation of oxygen free radicals generated during vanadate-stimulated NADH oxidation. Mol Cell Biochem 111:33–40
Bencherif M, Lukas RJ (1992) Vanadate amplifies receptor-mediated accumulation of inositol tris-and tetrakis phosphatase activities. Neurosci Lett 134:157–160
Cantley LC Jr, Cantley LG, Josephson L (1978) A characterisation of vanadate interactions with the (Na, K)-ATPase. Mechanistic and regulatory implications. J Biol Chem 253: 7361–7368
Cantley LC, Aisen P Jr (1979) The fate of cytoplasmatic vanadium implication on (Na, K)-ATPase inhibition. J Biol Chem 254:1781–1784
Brichard SM, Henquin JC (1995) The role of vanadium in the management of diabetes. Trends Pharmacol Sci 16:265–270
Macara IG (1986) Activation of 45Ca2+ influx and 22Na+/H+ exchange by epidermal growth factor and vanadate in A431 cells is independent of phosphatidylinositol turnover and is inhibited by phorbol ester and diacylglycerol. J Biol Chem 261:9321–9327
Stern A, Yin X, Tsang S-S et al (1993) Vanadium as a modulator of cellular regulatory cascades and oncogene expression. Biochem Cell Biol 71:103–112
Roden M, Liener K, Fűrnsinn C et al (1993) Non-insulin like action of sodium orthovanadate in the isolated perfused liver of fed non-diabetic rats. Diabetologia 36:602–607
Tertrin-Clary C, DeLaLlosa-Hermier MP, Roy M et al (1992) Activation of phospholipase C by different effectors in rat placement cells. Cell Signal 4:727–732
Zick Y, Sagi-Eisenberg R (1990) A combination of hydrogen peroxide and vanadate concomitantly stimulates protein tyrosine phosphorylation and polyphosphoinositide breakdown in different cell lines. Biochemistry 29:10240–10245
Richelmi P, Mirabelli F, Salis A et al (1989) On the role of mitochondria in cell injury caused by vanadate-induced Ca2+ overload. Toxicology 57:29–44
Schmitz W, Scholz H, Erdmann E et al (1982) Effect of vanadium in the +5, +4 and +3 oxidation states on cardiac force of contraction, adenylatecyclase and (Na+ + K+)-ATPase activity. Biochem Pharmacol 31:3853–3860
Madsen KL, Porter VM, Fedorak RN (1994) Vanadium reduces sodium-dependent glucose transport and increases glycolytic activity in LLC-PK1 epithelia. J Cell Physiol 158:459–466
Pilkis SJ, El-Maghrabi MR (1988) Hormonal regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Biochem 57:755–783
Cupo MA, Donaldson WE (1987) Chromium and vanadium effects on glucose metabolism and lipid synthesis in the chick. Poult Sci 66(1):120–126
Sera M, Tanaka K, Morita T et al (1990) Increasing effect of vanadate on lipoprotein lipase activity in isolated rat fat pads. Arch Biochem Biophys 279(2):291–297
Coulombe RA Jr, Briskin DP, Keller RJ et al (1987) Vanadium dependent oxidation of pyridine nucleotide in rat liver microsomal membranes. Arch Biochem Biophys 255(2): 267–273
Hajjar JJ, Fucci JC, Rowe WA et al (1987) Effect of vanadate on amino acid transport in rat jejunam. Proc Soc Exp Biol Med 184:403–409
Leonard A, Gerber GB (1994) Mutagenecity, carcinogenecity and teratogenicity of vanadium compounds. Mutat Res 317:81–88
Kopf-Maier P, Wagner W, Hesse B et al (1981) Tumor inhibition by metallocenes: activity against leukemias and detection of the systemic effect. Eur J Cancer 17:665–669
Kopf-Maier P, Krahl D (1983) Tumor inhibition by metallocenes: ultrastructural localisation of titanium and vanadium, in treated tumor cells by electron energy loss spectroscopy. Chem Biol Interact 44:317–328
Kopf-Maier P (1982) Development of necroses virus, activation and giant cell formation after treatment of Ehrlich ascites tumor with metallocene dichloride. J Cancer Res Clin Oncol 103:145–164
Kopf-Maier P, Wagner W, Liss E (1981) Cytokinetic behaviour of Ehrlich ascites tumor after in vivo treatment with cis-diamminedichloroplatinum (II) and metallocene dichloride. J Cancer Res Clin Oncol 201:21–30
El-Naggar MM, El-Waseef AM, El-Halafawy KM et al (1998) Antitumor activities of vanadium (IV), manganese(IV), iron(III), cobalt(II) and copper(II) complexes of 2-methylaminopyridine. Cancer Lett 133:71–76
Scrivens PJ, Alaoui MA, Giannini G et al (2003) Cdc-25A inhibitory properties and antineoplastic activity of bisperoxovanadium analogues. Mol Cancer Ther 2:1053–1059
D’Cruz OJ, Uckun FM (2002) Metavan: a novel oxovanadium (IV) complex with broad spectrum anticancer activity. Expert Opin Investig Drugs 11:1829–1836
Navara CS, Benyumov A, Vassilev A et al (2001) Vanadocenes as potent anti-proliferative agents disrupting mitotic spindle formation in cancer cells. Anticancer Drugs 12(4):369–376
Bishayee A, Oinam S, Basu M et al (2000) Vanadium chemoprevention of 7, 12-dimethylbenz(a) anthracene-induced rat mammary carcinogenesis: probable involvement of representative hepatic phase I and II xenobiotic metabolizing enzymes. Breast Cancer Res Treat 63(2):133–145
Moore CJ, Tricomi WA, Gould MN (1986) Interspecies comparison of polycyclic aromatic hydrocarbon metabolism in human and rat mammary epithelial cells. Cancer Res 46:4946–4952
Ray RS, Roy S, Ghosh S et al (2001) Suppression of cell proliferation, DNA protein cross-links, and induction of apoptosis by vanadium in chemical rat mammary carcinogenesis. Anticancer Drugs 12(4):369–376
Ray RS, Roy S, Samanta S et al (2005) Protective role of vanadium on the early process of rat mammary carcinogenesis by influencing expression of metallothionein. GGT-positive foci and DNA fragmentation. Cell Biochem Funct 23(6):447–456
Ray RS, Basu M, Ghosh B et al (2005) Vanadium, a versatile biochemical effector in chemical rat mammary carcinogenesis. Nutr Cancer 51(2):184–196
Ray RS, Rana B, Swami B et al (2006) Vanadium mediated apoptosis and cell cycle arrest in MCF7 cell line. Chem Biol Interact 63(3):239–247
Ray RS, Ghosh B, Rana A et al (2006) Suppression of cell proliferation, induction of apoptosis and cell cycle arrest: chemopreventive activity of vanadium in vivo and in vitro. Int J Cancer 120(1):13–23
Bishayee A, Chatterjee M (1993) Selective enhancement of glutathione S-transferase activity in liver and extrahepatic tissues of rat following oral administration of vanadate. Acta Physiol Pharmacol Bulg 19(3):83–89
Posner BI, Faure R, Burgess JW et al (1994) Peroxovanadium compounds. A new class of potent phosphotyrosine phosphatase inhibitors which are insulin mimetics. J Biol Chem 269(6):4596–4604
Bishayee A, Chatterjee M (1995) Inhibitory effect of vanadium on rat liver carcinogenesis initiated with diethylnitrosamine and promoted by phenobarbital. Br J Cancer 71(6): 1214–1220
Bishayee A, Karmakar R, Mandal A et al (1997) Vanadium-mediated chemoprotection against chemical hepatocarcinogenesis in rats: haematological and histological characteristics. Eur J Cancer Prev 6(1):58–70
Bishayee A, Roy S, Chatterjee M (1999) Characterization of selective induction and alteration of xenobiotic biotransforming enzymes by vanadium during diethylnitrosamine-induced chemical rat liver carcinogenesis. Oncol Res 11(1):41–53
Chakraborty A, Selvaraj S (2000) Differential modulation of xenobiotic metabolizing enzymes by vanadium during diethylnitrosamine-induced hepatocarcinogenesis in Sprague-Dawley rats. Neoplasma 47(2):81–89
Basak R, Saha BK, Chatterjee M (2000) Inhibition of diethylnitrosamine-induced rat liver chromosomal aberrations and DNA-strand breaks by synergistic supplementation of vanadium and 1alpha,25-dihydroxyvitamin D(3). Biochim Biophys Acta 1502(2):273–282
Basak R, Chatterjee M (2000) Combined supplementation of vanadium and 1alpha,25-dihydroxyvitamin D3 inhibit placental glutathione S-transferase positive foci in rat liver carcinogenesis. Life Sci 68(2):217–231
Basak R, Basu M, Chatterjee M (2000) Combined supplementation of vanadium and 1alpha,25-dihydroxyvitamin D(3) inhibit diethylnitrosamine-induced rat liver carcinogenesis. Chem Biol Interact 128(1):1–18
Bukietyńska K, Podsiadly H, Karwecka Z (2003) Complexes of vanadium(III) with L-alanine and L-aspartic acid. J Inorg Biochem 94(4):317–325
Osińska-Królicka I, Podsiadły H, Bukietyńska K et al (2004) Vanadium(III) complexes with L-cysteine-stability, speciation and the effect on actin in hepatoma Morris 5123 cells. J Inorg Biochem 98(12):2087–2098
Chakraborty T, Ghosh S, Datta S et al (2003) Vanadium suppresses sister-chromatid exchange and DNA-protein crosslink formation and restores antioxidant status and hepatocellular architecture during 2-acetylaminofluorene-induced experimental rat hepatocarcinogenesis. J Exp Ther Oncol 3(6):346–362
Chattopadhyay MB, Mahendrakumar CB, Kanna PS et al (2004) Combined supplementation of vanadium and beta-carotene suppresses placental glutathione S-transferase-positive foci and enhances antioxidant functions during the inhibition of diethylnitrosamine-induced rat liver carcinogenesis. J Gastroenterol Hepatol 19(6):683–693
Chakraborty T, Chatterjee A, Saralaya MG et al (2006) Vanadium inhibits the development of 2-acetylaminofluorene-induced premalignant phenotype in a two-stage chemical rat hepatocarcinogenesis model. Life Sci 78(24):2839–2851
Chakraborty T, Chatterjee A, Saralaya MG et al (2006) Chemopreventive effect of vanadium in a rodent model of chemical hepatocarcinogenesis: reflections in oxidative DNA damage, energy-dispersive X-ray fluorescence profile and metallothionein expression. J Biol Inorg Chem 11(7):855–866
Chakraborty T, Chatterjee A, Dhachinamoorthi D et al (2006) Vanadium limits the expression of proliferating cell nuclear antigen and inhibits early DNA damage during diethylnitrosamine-induced hepatocellular preneoplasia in rats. Environ Mol Mutagen 47(8):603–615
Chakraborty T, Pandey N, Chatterjee A et al (2006) Molecular basis of anticlastogenic potential of vanadium in vivo during the early stages of diethylnitrosamine-induced hepatocarcinogenesis in rats. Mutat Res 609(2):117–128
Chakraborty T, Chatterjee A, Rana A et al (2007) Carcinogen-induced early molecular events and its implication in the initiation of chemical hepatocarcinogenesis in rats: chemopreventive role of vanadium on this process. Biochim Biophys Acta 1772(1):48–59
Chakraborty T, Swamy AH, Chatterjee A et al (2007) Molecular basis of vanadium-mediated inhibition of hepatocellular preneoplasia during experimental hepatocarcinogenesis in rats. J Cell Biochem 101(1):244–258
Chakraborty T, Chatterjee A, Rana A et al (2007) Suppression of early stages of neoplastic transformation in a two-stage chemical hepatocarcinogenesis model: supplementation of vanadium, a dietary micronutrient, limits cell proliferation and inhibits the formations of 8-hydroxy-2′-deoxyguanosines and DNA strand-breaks in the liver of sprague-dawley rats. Nutr Cancer 59(2):228–247
Kordowiak AM, Klein A, Goc A et al (2007) Comparison of the effect of VOSO4, Na3VO4 and NaVO3 on proliferation, viability and morphology of H35-19 rat hepatoma cell line. Pol J Pathol 58(1):51–57
Greenle RT, Murry T, Wingo PA (2000) Cancer statistics, 2000. CA Cancer J Clin 50:7–33
Kingsnorth AN, LaMuraglia GM, Ross JS et al (1986) Vanadate supplements and 1, 2-dimethylhydrazine induced colon cancer in mice: increased thymidine incorporation without enhanced carcinogenesis. Br J Cancer 53(5):683–686
Kanna PS, Mahendrakumar CB, Chakraborty T et al (2003) Effect of vanadium on colonic aberrant crypt foci induced in rats by 1, 2 dimethyl hydrazine. World J Gastroenterol 9(5):1020–1027
Kanna PS, Mahendrakumar CB, Chatterjee M et al (2003) Vanadium inhibits placental glutathione S-transferase (GST-P) positive foci in 1,2-dimethyl hydrazine induced rat colon carcinogenesis. J Biochem Mol Toxicol 17(6):357–365
Kanna PS, Mahendrakumar CB, Indira BN et al (2004) Chemopreventive effects of vanadium toward 1,2-dimethylhydrazine-induced genotoxicity and preneoplastic lesions in rat colon. Environ Mol Mutagen 44(2):113–118
Kanna PS, Saralaya MG, Samanta K et al (2005) Vanadium inhibits DNA-protein cross-links and ameliorates surface level changes of aberrant crypt foci during 1,2-dimethylhydrazine induced rat colon carcinogenesis. Cell Biol Toxicol 21(1):41–52
Tan EY, Richard CL, Zhang H et al (2006) Adenosine downregulates DPPIV on HT-29 colon cancer cells by stimulating protein tyrosine phosphatase(s) and reducing ERK1/2 activity via a novel pathway. Am J Physiol Cell Physiol 291(3):C433–C444
Etcheverry SB, Ferrer EG, Naso L et al (2008) Antioxidant effects of the VO(IV) hesperidin complex and its role in cancer chemoprevention. J Biol Inorg Chem 13(3):435–447
Samanta S, Swamy V, Suresh D et al (2008) Protective effects of vanadium against DMH-induced genotoxicity and carcinogenesis in rat colon: removal of O(6)-methylguanine DNA adducts, p53 expression, inducible nitric oxide synthase downregulation and apoptotic induction. Mutat Res 650(2):123–131
Samanta S, Chatterjee M, Ghosh B et al (2008) Vanadium and 1, 25 (OH)2 vitamin D3 combination in inhibitions of 1,2, dimethylhydrazine-induced rat colon carcinogenesis. Biochim Biophys Acta 1780(10):1106–1114
Holko P, Ligeza J, Kisielewska J et al (2008) The effect of vanadyl sulphate (VOSO4) on autocrine growth of human epithelial cancer cell lines. Pol J Pathol 59(1):3–8
Klein A, Holko P, Ligeza J et al (2008) Sodium orthovanadate affects growth of some human epithelial cancer cells (A549, HTB44, DU145). Folia Biol (Krakow) 56(3–4):115–121
Papaioannou A, Manos M, Karkabounas S et al (2004) Solid state and solution studies of a vanadium(III)-L-cysteine compound and demonstration of its antimetastatic, antioxidant and inhibition of neutral endopeptidase activities. J Inorg Biochem 98(6):959–968
Hierowski MT, Liebow C, Schally AV et al (1985) Stimulation by somatostatin of dephosphorylation of membrane proteins in pancreatic cancer MIA PaCa-2 cell line. FEBS Lett 179(2):252–256
Cortizo MS, Alessandrini JL, Etcheverry SB et al (2001) A vanadium/aspirin complex controlled release using a poly(beta-propiolactone) film. Effects on osteosarcoma cells. J Biomater Sci Polym Ed 12(9):945–959
Cortizo AM, Bruzzone L, Molinuevo S et al (2000) A possible role of oxidative stress in the vanadium-induced cytotoxicity in the MC3T3E1 osteoblast and UMR106 osteosarcoma cell lines. Toxicology 147(2):89–99
Molinuevo MS, Cortizo AM, Etcheverry SB (2008) Vanadium(IV) complexes inhibit adhesion, migration and colony formation of UMR106 osteosarcoma cells. Cancer Chemother Pharmacol 61(5):767–773
Fallico R, Ferrante M, Fiore M et al (1998) Epidemiological research into the consequences of vanadium assimilated through diet and of its effects on human health following research carried out on people from the Etna massif. J Prev Med Hyg 39:74–79
Lener J, Kučera J, Kodl M et al (1998) Health effects of environmental exposure to vanadium. In: Nriagu JO (ed) Vanadium in the environment. Part 2: health effects. Wiley, New York
Greenwald P, Milner JA, Anderson DE et al (2002) Micronutrients in cancer chemoprevention. Cancer Metastasis Rev 21:217–230
Evangelou AM (2002) Vanadium in cancer treatment. Crit Rev Oncol Hematol 42:249–265
Bishayee A, Banik S, Marimuthu P et al (1997) Vanadium-mediated suppression of diethylnitrosamine-induced chromosomal aberrations in rat hepatocytes and its correlation with induction of hepatic glutathione and glutathione-S-transferase. Int J Oncol 102:413–423
Crans DC, Rithner CD, Theisen LA (1990) Application of time-resolved vanadium-51 2D NMR for quantitation of kinetic exchange pathways between vanadate monomer, dimer, tetramer, and pentamer. J Am Chem Soc 112:2901–2908
Heath E, Howarth OW (1981) Vanadium-51 and oxygen-17 nuclear magnetic resonance study of vanadate(V) equilibria and kinetics. J Chem Soc Dalton 1981:1105–1110
Hamilton EE, Wilker JJ (2004) Inorganic oxo compounds reacts with alkaline agents: implication for DNA damage. Angew Chem Int Ed Engl 43(25):3290–3292
Kawabe K, YoshikawaY AY et al (2006) Possible mode of action for insulin mimetic activity of vanadyl (IV) compounds in adipocytes. Life Sci 78:2860–2866
Zhang Z, Leonard SS, Huang C et al (2003) Role of oxygen species and MAPKs in vanadate- induced G2/M phase arrest. Free Radic Biol Med 34:1333–1342
Molinuevo MS, Barrio DA, Cortizo AM et al (2004) Antitumoral properties of two newvanadyl(IV) complexes in culture: role of apoptosis and oxidative stress. Cancer Chemother Pharmacol 53:163–172
Fawcett JP, Farquhar SJ, Thou T et al (1997) Oral vanadyl sulphate does not affect blood cells, viscosity or biochemistry in humans. Pharmacol Toxicol 80:202–206
Goldfine AB, Simonson DC, Folli F et al (1995) Metabolic effects of sodium metavanadate in humans with insulin-dependent and noninsulin-dependent diabetes mellitus in vivo and in vitro studies. J Clin Endocrinol Metab 80:3311–3320
Somerville J, Davies B (1962) Effect of vanadium on serum cholesterol. Am Heart J 64:54–56
Dimond EG, Caravace J, Benchimol A (1963) Vanadium, excretion, toxicity, lipid effect in man. Am J Clin Nutr 12:49–53
Schroeder HA, Balassa JJ, Tipton IH (1963) Abnormal trace metals in man- Vanadium. J Chronic Dis 16:1047–1071
Nemery B (1990) Metal toxicity and the respiratory tract. Eur Respir J 3(2):202–219
Ehrlich VA, Nersesyan AK, Atefie K et al (2008) Inhalative exposure to vanadium pentoxide causes DNA damage in workers: results of a multiple end point study. Environ Health Perspect 116(12):1689–1693
Maines MD (1994) Modulating factors that determine interindividual differences in response to metals. In: Mertz W et al (eds) Risk assessment of essential elements. ILSI Press, Washington, DC, pp 21–39
Acknowledgement
We thank Subrata Pramanik (M. Pharm.), Rohullah Roien (M. Pharm.) and Dr. Kaushik Roy for their continuous help and support for the preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Das, S., Chatterjee, M., Janarthan, M., Ramachandran, H., Chatterjee, M. (2012). Vanadium in Cancer Prevention. In: Michibata, H. (eds) Vanadium. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-0913-3_8
Download citation
DOI: https://doi.org/10.1007/978-94-007-0913-3_8
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-0912-6
Online ISBN: 978-94-007-0913-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)