Abstract
The distribution of radiocesium within trees in the forests of Mimamisoma, Fukushima, Japan, was studied between 2012 and 2013 after the Fukushima Nuclear Power Plant accident. Most of the radiocesium was contained in the foliage and bark of the examined trees of Japanese cedar (Cryptomeria japonica) and Japanese red pine (Pinus densiflora), although considerable concentrations were detected in the xylem of C. japonica. At higher positions in the trunk, there was more radiocesium in heartwood than in sapwood. Radiocesium in the xylem of a tree with its root system removed before the nuclear accident suggests that most of the radiocesium was not transferred through the root system but was likely translocated via the foliage.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
13.1 Introduction
The Fukushima Daiichi Nuclear Power Plant accident in March 2011 caused massive emissions of radioactive substances into the atmosphere and subsequently over a wide area of forests. Although many reports have examined nuclear materials within trees after the accident (Kuroda et al. 2013; Akama et al. 2013; Ohashi et al. 2014), the number of samples has been limited, and an accurate understanding requires an increased sample size. Since 2012, we have been measuring the radiocesium concentrations in trees in Minamisoma City, north of the nuclear power plant. The measurements have been conducted in cooperation with the Minamisoma City Office and the Soso District Agriculture and Forestry Office, Fukushima Prefecture. The city covers an area of 399 km2, of which 218 km2 is covered by forest. Approximately half of the forest is artificially planted for timber production. This study concentrated on two timber tree species, Japanese cedar (Cryptomeria japonica), and Japanese red pine (Pinus densiflora), which make up 3/4 of the standing timber volume of the forest.
13.2 Study Sites and Measurement of Radiocesium
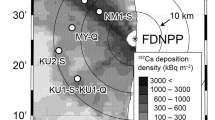
We investigated five forests stands owned and managed by Minamisoma City, locating 20–35 km NNW of the Fukushima Daiichi Nuclear Power Plant. All stands contained 50–60-year-old plantation forests.
According to the airborne monitoring map by Ministry of Education, Culture, Sports, Science and Technology, the minimum level of cesium 137 (137Cs) at all sites was 300 kBq/m2 and the maximum was estimated to be up to 3000 kBq/m2 on April 29, 2011. As an indicator of radiocesium deposition at each site at the time of sampling, we measured the air dose rate 1 m above the ground and close to the trees using a NaI scintillation survey meter.
The radionuclides were quantified in each sample using a germanium semiconductor detector. Peaks corresponding to 134Cs and 137Cs were detected for each sample. As 134Cs decayed naturally from 2012 to 2013, 137Cs levels were used in the present chapter to examine the radioactivity over the 2 years.
13.3 Distribution of Radiocesium in Standing Trees
Three trees, two C. japonica and one P. densiflora, were cut down in December 2012 (21 months after the accident) and in December 2013 (33 months after the accident), and the distribution of radiocesium on the inside and outside of the trees was investigated (Table 13.1). Among the six trees, five trees (#4 to #8) were felled from the same stand, and a C. japonica tree (#3) was felled from a stand with a comparatively higher air dose rate. Both stands were on southern facing slopes and were thinned in 2009.
To prevent soil particles contaminating the trunk after felling, each trunk was covered with a plastic sheet to a height of 1.5 m and the tree was cut close to the ground (Fig. 13.1A). After cutting, branches and foliage that did not touch the soil were sampled. The sample logs for analysis were taken at seven positions of each trunk in 2012 and at 2–3 positions in 2013 (Fig. 13.1B). The whole tree was weighed, including the parts not sampled.
The sample logs were transported to a sawmill and cut into 5 cm disks with a bandsaw. The logs harvested in 2012 were subsequently separated into bark, heartwood, and sapwood. The logs harvested in 2013 were stripped of the bark at the felling site, samples of the bark were taken, and the remaining bark fragments were brushed away. Subsequently, disks composed of xylem were cut and divided into sapwood and heartwood. In addition, for a C. japonica sample in 2013, the pale-colored rings between sapwood and heartwood were further sampled separately as transition wood. The woods were cleaved into 5-cm long and 0.2–1-cm thick fragments, dried, and analyzed to determine their 137Cs levels.
The stumps of C. japonica #3 and P. densiflora #7 were pulled out of the ground with a grapple machine. Lateral roots of 4–7 cm diameter were sampled, and the xylem with the bark removed was analyzed for 137Cs.
There was a large variation in radiocesium levels among the trees and within each tree depending on the sampled positions. The highest concentrations occurred in the foliage followed by the bark of the trunk (Tables 13.1 and 13.2). Radiocesium was detected in the xylem of all logs (Table 13.1).
The total volume of radiocesium in each organ was calculated by multiplying the radiocesium concentration by the dry weight. In 2012, less than 20 % of the radiocesium from the aboveground part was in the trunk; most was in the foliage. In 2013, up to 40 % of radiocesium was in the trunk. The change in the distribution of radiocesium was probably due to gradual defoliation of foliage that was on the trees at the time of the accident.
To increase the sample size, wood samples were taken without felling from trunks with a band drill from 11 C. japonica, three P. densiflora, and one Japanese cypress (Chamaecyparis obtusa) at three stands.
A 7 cm × 7 cm sample of bark was peeled from each sample tree at a breast height using a knife and a handsaw. The exposed xylem was brushed and then drilled in the radial direction with a hand drill of an 18 mm drill bit and a clickball. The shavings from the drilling operation were collected in a plastic bag (Fig. 13.1C). The sapwood from C. japonica and C. obtusa was collected until the shavings became darker, and subsequent shavings were heartwood samples. The heartwood and sapwood of P. densiflora was harder to distinguish on the basis of color; therefore, both were collected together without separation. Three to five holes were drilled to provide sufficient samples for analysis.
The radiocesium concentration was high in the bark of all trees and it was also detected in the xylem. Although the radiocesium levels in the trees tended to be higher in the stand with the higher air dose rate, there was also a large variation between the trees in each stand (Fig. 13.2). It may be possible to estimate the amount of radiocesium in the xylem from the air dose rate and to estimate the cesium deposition, but the large variation between trees should be noted.
The distribution of radiocesium within 5-mm thick disks cut from the same logs used for quantitative measurement of radiocesium was visualized using an imaging plate (BAS-IP MS, GE Healthcare Japan). Disks and image plates were stacked in the cassette and kept in a box made of ≥5-cm thick lead bricks to shield against natural radiation. Images were obtained using a fluorescent image analyzer (FLA-9000, Fujifilm) after the exposure for 1–5 months. Disks were also taken from C. japonica and P. densiflora trees felled at the Ecohydrology Research Institute, University of Tokyo, 425 km southeast of the Fukushima Daiichi nuclear power plant, and were exposed to the image plates under the same conditions as described above. However, no images could be detected from these log disks. This indicates that the images obtained from the Minamisoma samples represented the distribution of radioactive substances emitted from the nuclear power plant accident.
The images from each log disk are shown in Fig. 13.3. Similar to the results from Table 13.1, C. japonica have higher radiocesium concentrations in the heartwood than in the sapwood, especially for disks that were taken from higher positions nearer the crown. Kuroda et al. (2013) reported higher concentrations in C. japonica sapwood than heartwood, but they only analyzed wood lower than 3 m; therefore, their results do not necessarily conflict with ours. The imaging plates for the trunk xylem of C. japonica #5 show a dark color indicating the strong presence of radiocesium, which appeared to be highest at the outer edges of heartwood where heartwood formation was taking place (Fig. 13.3). For C. japonica #5 and #6, we analyzed transition wood with a low moisture content at the boundary between the heartwood and sapwood. The dry weight concentration of radiocesium was higher in the heartwood, followed by the transition wood, and then the sapwood (Table 13.1). In C. japonica, rubidium is actively transported from the sapwood to the outer heartwood via xylem ray (Okada et al. 2012), indicating that this mechanism could be used to transport another alkaline metals such as cesium.
Trunk cross-sections showing distribution of radioactivity. Five sample trees cut into log disks at various heights (right) were exposed to imaging plates (Masumori et al. 2015). Darker colors indicate higher radioactivity. The log disks included the bark for #3, #4, and #7. The black spots on the xylem image are due to some scattering of bark fragments. The bark from C. japonica #5 and #6 was peeled off before cutting the log disks; therefore, there is no interference from bark fragments. The bark from P. densiflora #8 was stripped off in the same way, but no radiation was seen (data not shown)
The concentration of radiocesium was less in the xylem of P. densiflora than C. japonica and less in the heartwood than the sapwood of both the felled trees and the cored samples (Fig. 13.2). Samples of C. obtusa, another timber species, were taken from only one tree, but the radiocesium distribution in the xylem was similar to C. japonica. If the radiocesium was initially absorbed into the xylem via the foliage, then there should be a relationship between the foliage volume and radiocesium amount in the xylem. For the five trees felled from the same stand (#4–#8), the total radiocesium amount in the xylem of each trunk was compared with the dry weight of the foliage (as an index of total foliage volume) to test whether there was a correlation. The radiocesium amount in the trunk xylem was proportional to the 2/3 power of foliage dry weight, which indicates a surface area (Fig. 13.4). The interspecific differences in radiocesium content between the xylem of C. japonica and P. densiflora were not related to the anatomical or physiological characters of each species but were more likely due to differences in the areal quantity of the foliage surface where radiocesium deposited.
Total radiocesium content in the xylem of trees and the dry weight of the foliage from a single stand (Masumori et al. 2015)
13.4 Distribution of Radiocesium in the Crown
Radiocesium was analyzed in the leaves of different ages. From the felled P. densiflora trees, we separately sampled the needles from current-year shoots and needles from shoots elongated in the previous year. On the sampling day in December, the pine had no needles on the older shoots. Young C. japonica shoots are densely covered with needle-like leaves; thus, the leaves and stem were not separated. The remaining shoots comprising leaves and stem were separated according to the year of growth, up to 3 years old. Shoots more than 3 years old were counted as branches, even if they still had needles attached.
Branches that developed before the accident in March 2011 showed the highest radiocesium concentrations, although radiocesium was detected in foliage that had developed after the accident (Table 13.2).
Eight branches were analyzed separately from C. japonica #5. A large variation in radiocesium concentration occurred between the branches and the foliage (Table 13.2). In agreement with Akama et al. (2013), we found that for each branch, the younger leaves tended to have the lowest radiocesium concentrations, but the concentrations in the older leaves did not correlate with those in the younger leaves (Table 13.2). Therefore, the movement of radiocesium to newly elongating shoots of the same branch was not necessarily driven by a concentration gradient. The youngest leaves on branches at a lower position on the tree had higher concentrations of radiocesium than those on higher branches. The youngest leaves of C. japonica #6 had lower positioned foliage and higher radiocesium concentration than those of C. japonica #5 (Table 13.2, Fig. 13.5). Although most radiocesium in the older foliage was immobilized, some radiocesium is likely to be mobile and translocated not only within the tree but also outside the tree and downwards through the forest canopy such as with rain. We suggest that the position in the canopy should be taken into account when considering radiocesium migration into developing organs.
Radiocesium content in C. japonica leaves developed after the nuclear accident. Data from Table 13.3 is included. The value at height of 11.1 m is for Tree #6. All other values are for Tree #5
13.5 Radiocesium in Xylem from Fallen Trees
In August 2012, samples were taken from the trunks of two C. japonica trees that had already fallen and their radiocesium concentrations were measured (Table 13.3). At the forest site where the two trees had grown, thinning was occurring at the time of the earthquake on March 11, 2011. Tree #1 had been felled, but work was interrupted because of the earthquake before limbing procedure and the tree was left on the forest floor with the foliage still attached. Tree #2 had been growing in the vicinity of tree #1 but had not been thinned, although it was uprooted during the typhoon in June 2012 and had been lying on the forest floor for a month and a half at the time of our research. Consequently, tree #1 would have been separated from its roots at the time of the nuclear power plant accident, but tree #2 would have been intact and growing.
Two disks were sampled from the logs of each tree at 1.3 m and 13 m from the ground. At these positions, the trunk had not touched the soil, even after the tree had fallen. From each disk, the bark was sampled and the xylem was separated into six fractions from the sapwood to the pith according to the tree rings. For tree #1, separate cesium analyses were conducted for the semicircular half of lying trunk facing the sky and the semicircular half of the lying trunk facing the ground.
Radiocesium was present in the xylem of the trunk of C. japonica #1, which had been separated from its roots at the time of the nuclear power plant accident. C. japonica #2 had similar radiocesium concentrations in the xylem, although it had been an intact tree up to 15 months after the accident (Table 13.3). This suggests that most of the radiocesium in the xylem of C. japonica is not absorbed through the roots. The concentration of radiocesium in the roots of C. japonica #3 was lower than that in the trunk (Table 13.1), suggesting that a comparatively small amount of radiocesium had migrated from the roots to the trunk at the time of sampling.
If the radiocesium emitted by the accident had precipitated with the rain, the amount of radiocesium deposited on the sky-facing and ground-facing of the fallen C. japonica #1 should differ. Higher concentrations of radiocesium were detected in the sky-facing bark (Table 13.3). If radiocesium diffused inward from the bark to the xylem, there should be a higher concentration in the sky-facing xylem, but in contrast to the bark, there were no clear differences between sky-facing and ground-facing xylem (Table 13.3). Although C. japonica #1 was felled, the foliage remained in place; thus, physiological activity would have continued at the tissue level. Therefore, the radiocesium detected in the xylem of the trunk may have migrated basipetally through the vascular bundle from deposits on the foliage.
13.6 Greenhouse Experiments
Greenhouse cultivation experiments were conducted to determine the characteristics of radiocesium absorption from the roots in both C. japonica and P. densiflora. These experiments also allowed us to examine the effects of secondary deposits from the canopy, which contained a high quantity of radioactive deposition, and migration from resuspended soil particles.
Two-year-old seedlings grown in a radiocesium-free environment were transplanted in pots with soil from Minamisoma forests containing 20 Bq/g of 137Cs. After 5 months in the greenhouse, shoots from three C. japonica seedlings and seven P. densiflora seedlings were analyzed for radiocesium. Because a 5-month period was insufficient for the C. japonica plants to recover an appropriate contact between root system and potting media, not much shoot growth was seen during this period. 137Cs migration into the C. japonica shoots was 5–10 Bq/kg, and migration into the P. densiflora shoots was 2–38 Bq/kg. Imaging plates show that the radioactivity was uniformly distributed among all the organs within the shoots, except it was higher in newly grown P. densiflora shoots (Fig. 13.6).
Radiocesium in seedlings absorbed through the roots. Cryptomeria japonica (top) and Pinus densiflora (bottom) (Masumori et al. 2014). The seedlings were planted in a greenhouse in potting medium rich in radiocesium (right panels). The shoots were exposed to imaging plates 5 months later to visualize the radiocesium distribution (left panels)
In standing trees, the incorporated radiocesium in the xylem was greater in C. japonica than in P. densiflora, while migration through the roots did not differ between the species. In these forests, the levels of radiocesium in the xylem can gradually increase because of absorption via the root from the forest floor where much of the radiocesium is deposited and is accumulating.
References
Akama A, Kiyono Y, Kanazashi T, Shichi K (2013) Survey of radioactive contamination of sugi (C. japonica japonica D. Don) shoots and male flowers in Fukushima prefecture. Jpn J For Environ 55:105–111
Kuroda K, Kagawa A, Tonosaki M (2013) Radiocesium concentrations in the bark, sapwood and heartwood of three tree species collected at Fukushima forests half a year after the Fukushima Dai-ichi nuclear accident. J Environ Radioact 122:37–42
Masumori M, Nogawa N, Tange T (2014) Distribution of radiocesium released from the NPP accident within trees – cases of forests in Minamisoma, Fukushima. Tree For Health 18:70–71 (in Japanese)
Masumori M, Nogawa N, Sugiura S, Tange T (2015) Radiocesium in stem, branch and leaf of Cryptomeria japonica and Pinus densiflora trees: cases of forests in Minamisoma in 2012 and 2013. J Jpn For Soc 97:51–56 (in Japanese with English summary)
Ohashi S, Okada N, Tanaka A, Nakai W, Takano S (2014) Radial and vertical distributions of radiocesium in tree stems of P. densiflora densiflora and Quercus serrata 1.5 y after the Fukushima nuclear disaster. J Environ Radioact 134:54–60
Okada N, Hirakawa Y, Katayama Y (2012) Radial movement of sapwood-injected rubidium into heartwood of Japanese cedar (C. japonica japonica) in the growing period. J Wood Sci 58:1–8
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Copyright information
© 2016 The Author(s)
About this chapter
Cite this chapter
Masumori, M., Nogawa, N., Sugiura, S., Tange, T. (2016). Radiocesium in Timber of Japanese Cedar and Japanese Red Pine, in the Forests of Minamisoma, Fukushima. In: Nakanishi, T., Tanoi, K. (eds) Agricultural Implications of the Fukushima Nuclear Accident. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55828-6_13
Download citation
DOI: https://doi.org/10.1007/978-4-431-55828-6_13
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55826-2
Online ISBN: 978-4-431-55828-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)