Abstract
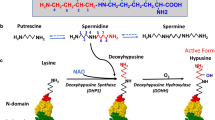
Although polyamines exert various effects on nucleic acids and macromolecular synthesis as polycations, spermidine is covalently incorporated into a single protein, eukaryotic initiation factor 5A (eIF5A), through a unique posttranslational modification. In this reaction, the aminobutyl moiety of spermidine is conjugated to a specific lysine residue of eIF5A to form an unusual amino acid, hypusine [N ε-(4-amino-2-hydroxybutyl)-lysine]. It occurs by two enzymatic steps catalyzed by deoxyhypusine synthase (DHS) and deoxyhypusine hydroxylase (DOHH). Hypusine synthesis occurs exclusively in eIF5A and is essential for eukaryotic cell proliferation. Although only a small percentage of the total spermidine in cells is used for hypusine formation, cells cannot survive/grow when hypusinated eIF5A falls below a critical level. Inactivation of the eIF5A gene or DHS gene is lethal in yeast and in mouse, further indicating the vital role of hypusinated eIF5A. eIF5A has been proposed to promote translation of a subset of cellular mRNAs. Indeed, recent evidence suggests that eIF5A facilitates translation at the elongation step, particularly at multiple strings of proline residues. A model of eIF5A docked in the ribosome reveals the hypusine directed toward the peptidyl transferase center. Thus, the hypusine modification defines a link between polyamines and cell growth, through promotion of translation.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Notes
- 1.
Approved anti-fungal drug.
- 2.
Approved anti-thalassemia drug.
Abbreviations
- DHS :
-
Deoxyhypusine synthase
- DOHH :
-
Deoxyhypusine hydroxylase
- EF-P:
-
Bacterial elongation factor P
- eIF5A :
-
Eukaryotic initiation factor 5A
- GC7:
-
N1-guanyl-1,7-diaminoheptane
- SSAT1:
-
Spermidine /spermine acetyltransferase 1
References
Byers TL, Ganem B, Pegg AE (1992) Cytostasis induced in L1210 murine leukaemia cells by the S-adenosyl-l-methionine decarboxylase inhibitor 5′-([(Z)-4-amino-2-butenyl]methylamino)-5′-deoxyadenosine may be due to hypusine depletion. Biochem J 287:717–724
Byers TL, Lakanen JR, Coward JK et al (1994) The role of hypusine depletion in cytostasis induced by S-adenosyl-l-methionine decarboxylase inhibition: new evidence provided by 1-methylspermidine and 1,12-dimethylspermine. Biochem J 303:363–368
Cano VS, Jeon GA, Johansson HE et al (2008) Mutational analyses of human eIF5A–1: identification of amino acid residues critical for eIF5A activity and hypusine modification. FEBS J 275:44–58
Caraglia M, Park MH, Wolff EC et al (2013) eIF5A isoforms and cancer: two brothers for two functions? Amino Acids 44:103–109
Chattopadhyay MK, Park MH, Tabor H (2008) Hypusine modification for growth is the major function of spermidine in Saccharomyces cerevisiae polyamine auxotrophs grown in limiting spermidine. Proc Natl Acad Sci USA 105:6554–6559
Chen ZP, Chen KY (1997a) Dramatic attenuation of hypusine formation on eukaryotic initiation factor 5A during senescence of IMR-90 human diploid fibroblasts. J Cell Physiol 170:248–254
Chen ZP, Chen KY (1997b) Marked elevation of hypusine formation activity on eukaryotic initiation factor 5A in v-HA-RAS transformed mouse NIH3T3 cells. Cancer Lett 115:235–241
Chen KY, Liu AY (1997) Biochemistry and function of hypusine formation on eukaryotic initiation factor 5A. Biol Signals 6:105–109
Clement PM, Hanauske-Abel HM, Wolff EC et al (2002) The antifungal drug ciclopirox inhibits deoxyhypusine and proline hydroxylation, endothelial cell growth and angiogenesis in vitro. Int J Cancer 100:491–498
Cooper HL, Park MH, Folk JE et al (1983) Identification of the hypusine-containing protein hy+ as translation initiation factor eIF-4D. Proc Natl Acad Sci USA 80:1854–1857
Doerfel LK, Wohlgemuth I, Kothe C et al (2013) EF-P is essential for rapid synthesis of proteins containing consecutive proline residues. Science 339:85–88
Gerner EW, Mamont PS, Bernhardt A et al (1986) Post-translational modification of the protein-synthesis initiation factor eIF-4D by spermidine in rat hepatoma cells. Biochem J 239:379–386
Gregio AP, Cano VP, Avaca JS et al (2009) eIF5A has a function in the elongation step of translation in yeast. Biochem Biophys Res Commun 380:785–790
Gutierrez E, Shin BS, Woolstenhulme CJ et al (2013) eIF5A promotes translation of polyproline motifs. Mol Cell 51:35–45
Hanauske-Abel HM, Park MH, Hanauske AR et al (1994) Inhibition of the G1-S transition of the cell cycle by inhibitors of deoxyhypusine hydroxylation. Biochim Biophys Acta 1221:115–124
Hyvonen MT, Keinanen TA, Cerrada-Gimenez M et al (2007) Role of hypusinated eukaryotic translation initiation factor 5A in polyamine depletion-induced cytostasis. J Biol Chem 282:34700–34706
Ishfaq M, Maeta K, Maeda S et al (2012) Acetylation regulates subcellular localization of eukaryotic translation initiation factor 5A (eIF5A). FEBS Lett 586:3236–3241
Jakus J, Wolff EC, Park MH et al (1993) Features of the spermidine-binding site of deoxyhypusine synthase as derived from inhibition studies. Effective inhibition by bis- and mono-guanylated diamines and polyamines. J Biol Chem 268:13151–13159
Jasiulionis MG, Luchessi AD, Moreira AG et al (2007) Inhibition of eukaryotic translation initiation factor 5A (eIF5A) hypusination impairs melanoma growth. Cell Biochem Funct 25:109–114
Kemper WM, Berry KW, Merrick WC (1976) Purification and properties of rabbit reticulocyte protein synthesis initiation factors M2Balpha and M2Bbeta. J Biol Chem 251:5551–5557
Kim SC, Sprung R, Chen Y et al (2006a) Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell 23:607–618
Kim YS, Kang KR, Wolff EC et al (2006b) Deoxyhypusine hydroxylase is a Fe(II)-dependent, HEAT-repeat enzyme. Identification of amino acid residues critical for Fe(II) binding and catalysis [corrected]. J Biol Chem 281:13217–13225
Klier H, Csonga R, Joao HC et al (1995) Isolation and structural characterization of different isoforms of the hypusine-containing protein eIF-5A from HeLa cells. Biochemistry 34:14693–14702
Lee SB, Park JH, Kaevel J et al (2009) The effect of hypusine modification on the intracellular localization of eIF5A. Biochem Biophys Res Commun 383:497–502
Lee SB, Park JH, Folk JE et al (2011) Inactivation of eukaryotic initiation factor 5A (eIF5A) by specific acetylation of its hypusine residue by spermidine/spermine acetyltransferase 1 (SSAT1). Biochem J 433:205–213
Mandal S, Mandal A, Johansson HE et al (2013) Depletion of cellular polyamines, spermidine and spermine, causes a total arrest in translation and growth in mammalian cells. Proc Natl Acad Sci USA 110:2169–2174
Nakajima T, Matsubayashi T, Kakimoto Y et al (1971) Distribution of hypusine, N 6-(4-amino-2-hydroxybutyl)-2,6-diaminohexanoic acid, in mammalian organs. Biochim Biophys Acta 252:92–97
Nishimura K, Murozumi K, Shirahata A et al (2005) Independent roles of eIF5A and polyamines in cell proliferation. Biochem J 385:779–785
Nishimura K, Lee SB, Park JH et al (2012) Essential role of eIF5A-1 and deoxyhypusine synthase in mouse embryonic development. Amino Acids 42:703–710
Park MH (2006) The post-translational synthesis of a polyamine-derived amino acid, hypusine, in the eukaryotic translation initiation factor 5A (eIF5A). J Biochem 139:161–169
Park MH, Cooper HL, Folk JE (1981) Identification of hypusine, an unusual amino acid, in a protein from human lymphocytes and of spermidine as its biosynthetic precursor. Proc Natl Acad Sci USA 78:2869–2873
Park MH, Wolff EC, Folk JE (1993) Hypusine: its post-translational formation in eukaryotic initiation factor 5A and its potential role in cellular regulation. Biofactors 4:95–104
Park MH, Wolff EC, Lee YB et al (1994) Antiproliferative effects of inhibitors of deoxyhypusine synthase. Inhibition of growth of Chinese hamster ovary cells by guanyl diamines. J Biol Chem 269:27827–27832
Park MH, Joe YA, Kang KR (1998) Deoxyhypusine synthase activity is essential for cell viability in the yeast Saccharomyces cerevisiae. J Biol Chem 273:1677–1683
Park JH, Aravind L, Wolff EC et al (2006) Molecular cloning, expression, and structural prediction of deoxyhypusine hydroxylase: a HEAT-repeat-containing metalloenzyme. Proc Natl Acad Sci USA 103:51–56
Park MH, Mandal S, Mandal A et al (2014) eIF5A and cancer. In: Parsyan A (ed) Translation and cancer: applications in medicine. Springer, New York, pp 223–232
Patel PH, Costa-Mattioli M, Schulze KL et al (2009) The Drosophila deoxyhypusine hydroxylase homologue nero and its target eIF5A are required for cell growth and the regulation of autophagy. J Cell Biol 185:1181–1194
Saini P, Eyler DE, Green R et al (2009) Hypusine-containing protein eIF5A promotes translation elongation. Nature (Lond) 459:118–121
Sasaki K, Abid MR, Miyazaki M (1996) Deoxyhypusine synthase gene is essential for cell viability in the yeast Saccharomyces cerevisiae. FEBS Lett 384:151–154
Schnier J, Schwelberger HG, Smit-McBride Z et al (1991) Translation initiation factor 5A and its hypusine modification are essential for cell viability in the yeast Saccharomyces cerevisiae. Mol Cell Biol 11:3105–3114
Shi XP, Yin KC, Ahern J et al (1996) Effects of N 1-guanyl-1,7-diaminoheptane, an inhibitor of deoxyhypusine synthase, on the growth of tumorigenic cell lines in culture. Biochim Biophys Acta 1310:119–126
Shiba T, Mizote H, Kaneko T et al (1971) Hypusine, a new amino acid occurring in bovine brain. Isolation and structural determination. Biochim Biophys Acta 244:523–531
Ude S, Lassak J, Starosta AL et al (2013) Translation elongation factor EF-P alleviates ribosome stalling at polyproline stretches. Science 339:82–85
Wang FW, Guan XY, Xie D (2013) Roles of eukaryotic initiation factor 5A2 in human cancer. Int J Biol Sci 9:1013–1020
Wolff EC, Kang KR, Kim YS et al (2007) Posttranslational synthesis of hypusine: evolutionary progression and specificity of the hypusine modification. Amino Acids 33:341–350
Zhou H, Shen T, Luo Y et al (2010) The antitumor activity of the fungicide ciclopirox. Int J Cancer 127:2467–2477
Acknowledgments
This research was supported by the Intramural Research Program of the NIH/NIDCR.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Japan
About this chapter
Cite this chapter
Wolff, E.C., Park, M.H. (2015). Role of the Polyamine Spermidine as a Precursor for Hypusine Modification in eIF5A. In: Kusano, T., Suzuki, H. (eds) Polyamines. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55212-3_10
Download citation
DOI: https://doi.org/10.1007/978-4-431-55212-3_10
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55211-6
Online ISBN: 978-4-431-55212-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)