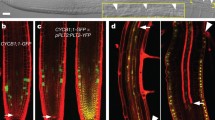
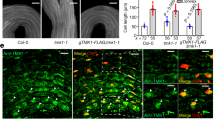
Abstract
The essential role of auxin for cell proliferation in plants is well known. Both auxin signaling and cell cycle regulation have been studied elaborately, but less is known about the connection between these processes. Recent studies report on the first molecular pathways that have been found to directly link auxin levels to the regulation of cell cycle activity. Here, we discuss the general effect of auxin on cell cycle progression and then zoom in on the interplay between auxin and the cell cycle during root development in Arabidopsis thaliana. At the root tip, an auxin gradient maintains the correct organization of the ground tissue layers and controls the size of the root apical meristem. During auxin-induced lateral root initiation LATERAL ORGAN BOUNDARIES-DOMAIN transcription factors are upregulated and control reactivation of the cell cycle and cell specification, both of which are needed for proper lateral root initiation. Auxin-induced lateral root initiation-like pathways are also involved in cell cycle reactivation during the formation of nematode feeding sites, nitrogen-fixing nodules and callus tissue, pointing to the existence of one common auxin–cell cycle module to initiate new organs in plants.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Aida M, Beis D, Heidstra R, Willemsen V, Blilou I, Galinha C, Nussaume L, Noh Y-S, Amasino R, Scheres B (2004) The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell 119(1):109–120. doi:10.1016/j.cell.2004.09.018
Atta R, Laurens L, Boucheron-Dubuisson E, Guivarc’h A, Carnero E, Giraudat-Pautot V, Rech P, Chriqui D (2009) Pluripotency of Arabidopsis xylem pericycle underlies shoot regeneration from root and hypocotyl explants grown in vitro. Plant J 57(4):626–644. doi:10.1111/j.1365-313X.2008.03715.x
Bayliss MW (1985) Regulation of the cell division cycle in cultured plant cells. In: Bryant JA, Francis D (eds) The cell division cycle in plants. Cambridge University Press, Cambridge, pp 157–177
Beeckman T, Burssens S, Inzé D (2001) The peri-cell-cycle in Arabidopsis. J Exp Bot 52(Spec Issue):403–411
Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115(5):591–602
Berckmans B, Vassileva V, Schmid SPC, Maes S, Parizot B, Naramoto S, Magyar Z, Alvim Kamei CL, Koncz C, Bögre L, Persiau G, De Jaeger G, Friml J, Simon R, Beeckman T, De Veylder L (2011) Auxin-dependent cell cycle reactivation through transcriptional regulation of Arabidopsis E2Fa by lateral organ boundary proteins. Plant Cell 23(10):3671–3683. doi:10.1105/tpc.111.088377
Blakely LM, Evans TA (1979) Cell dynamics studies on the pericycle of radish seedling roots. Plant Sci Lett 14(1):79–83
Boerjan W, Cervera M-T, Delarue M, Beeckman T, Dewitte W, Bellini C, Caboche M, Van Onckelen H, Van Montagu M, Inzé D (1995) superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell 7(9):1405–1419
Bradley MV, Crane JC (1955) The effect of 2,4,5-trichlorophenoxyacetic acid on cell and nuclear size and endopolyploidy in parenchyma of apricot fruits. Am J Bot 42(3):273–281
Burssens S, de Almeida Engler J, Beeckman T, Richard C, Shaul O, Ferreira P, Van Montagu M, Inzé D (2000) Developmental expression of the Arabidopsis thaliana CycA2;1 gene. Planta 211(5):623–631
Celenza JL Jr, Grisafi PL, Fink GR (1995) A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev 9(17):2131–2142
Chevalier C (2007) Cell cycle control and fruit development. In: Inzé D (ed) Cell cycle control and plant development, vol 32, Annual plant reviews. Blackwell, Oxford, UK
Chupeau M-C, Granier F, Pichon O, Renou J-P, Gaudin V, Chupeau Y (2013) Characterization of the early events leading to totipotency in an Arabidopsis protoplast liquid culture by temporal transcript profiling. Plant Cell. doi:10.1105/tpc.113.109538
Churchman ML, Brown ML, Kato N, Kirik V, Hülskamp M, Inzé D, De Veylder L, Walker JD, Zheng Z, Oppenheimer DG, Gwin T, Churchman J, Larkin JC (2006) SIAMESE, a plant-specific cell cycle regulator, controls endoreplication onset in Arabidopsis thaliana. Plant Cell 18(11):3145–3157. doi:10.1105/tpc.106.044834
Cools T, Iantcheva A, Weimer AK, Boens S, Takahashi N, Maes S, Van den Daele H, Van Isterdael G, Schnittger A, De Veylder L (2011) The Arabidopsis thaliana checkpoint kinase WEE1 protects against premature vascular differentiation during replication stress. Plant Cell 23(4):1435–1448. doi:10.1105/tpc.110.082768
Cruz-Ramírez A, Díaz-Triviño S, Blilou I, Grieneisen VA, Sozzani R, Zamioudis C, Miskolczi P, Nieuwland J, Benjamins R, Dhonukshe P, Caballero-Pérez J, Horvath B, Long Y, Mähönen AP, Zhang H, Xu J, Murray JAH, Benfey PN, Bako L, Marée AFM, Scheres B (2012) A bistable circuit involving SCARECROW-RETINOBLASTOMA integrates cues to inform asymmetric stem cell division. Cell 150(5):1002–1015. doi:10.1016/j.cell.2012.07.017
de Almeida Engler J, De Vleesschauwer V, Burssens S, Celenza JL Jr, Inzé D, Van Montagu M, Engler G, Gheysen G (1999) Molecular markers and cell cycle inhibitors show the importance of cell cycle progression in nematode-induced galls and syncytia. Plant Cell 11(5):793–808
de Almeida Engler J, De Veylder L, De Groodt R, Rombauts S, Boudolf V, De Meyer B, Hemerly A, Ferreira P, Beeckman T, Karimi M, Hilson P, Inzé D, Engler G (2009) Systematic analysis of cell-cycle gene expression during Arabidopsis development. Plant J 59(4):645–660. doi:10.1111/j.1365-313X.2009.03893.x
De Rybel B, Vassileva V, Parizot B, Demeulenaere M, Grunewald W, Audenaert D, Van Campenhout J, Overvoorde P, Jansen L, Vanneste S, Möller B, Wilson M, Holman T, Van Isterdael G, Brunoud G, Vuylsteke M, Vernoux T, De Veylder L, Inzé D, Weijers D, Bennett MJ, Beeckman T (2010) A novel Aux/IAA28 signaling cascade activates GATA23-dependent specification of lateral root founder cell identity. Curr Biol 20(19):1697–1706. doi:10.1016/j.cub.2010.09.007, S0960-9822(10)01088-2 [pii]
De Schutter K, Joubès J, Cools T, Verkest A, Corellou F, Babiychuk E, Van Der Schueren E, Beeckman T, Kushnir S, Inzé D, De Veylder L (2007) Arabidopsis WEE1 kinase controls cell cycle arrest in response to activation of the DNA integrity checkpoint. Plant Cell 19(1):211–225. doi:10.1105/tpc.106.045047
De Smet I, Beeckman T (2011) Asymmetric cell division in land plants and algae: the driving force for differentiation. Nat Rev Mol Cell Biol 12(3):177–188. doi:10.1038/nrm3064
De Smet I, Tetsumura T, De Rybel B, Frei dit Frey N, Laplaze L, Casimiro I, Swarup R, Naudts M, Vanneste S, Audenaert D, Inzé D, Bennett MJ, Beeckman T (2007) Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development 134(4):681–690
De Smet I, Lau S, Voß U, Vanneste S, Benjamins R, Rademacher EH, Schlereth A, De Rybel B, Vassileva V, Grunewald W, Naudts M, Levesque MP, Ehrismann JS, Inzé D, Luschnig C, Benfey PN, Weijers D, Van Montagu MCE, Bennett MJ, Jürgens G, Beeckman T (2010) Bimodular auxin response controls organogenesis in Arabidopsis. Proc Natl Acad Sci U S A 107(6):2705–2710. doi:10.1073/pnas.0915001107
De Veylder L, de Almeida Engler J, Burssens S, Manevski A, Lescure B, Van Montagu M, Engler G, Inzé D (1999) A new D-type cyclin of Arabidopsis thaliana expressed during lateral root primordia formation. Planta 208(4):453–462
De Veylder L, Larkin JC, Schnittger A (2011) Molecular control and function of endoreplication in development and physiology. Trends Plant Sci 16(11):624–634. doi:10.1016/j.tplants.2011.07.001
del Pozo JC, Manzano C (2013) Auxin and the ubiquitin pathway. Two players-one target: the cell cycle in action. J Exp Bot. doi:10.1093/jxb/ert363
del Pozo JC, Boniotti MB, Gutierrez C (2002) Arabidopsis E2Fc functions in cell division and is degraded by the ubiquitin-SCFAtSKP2 pathway in response to light. Plant Cell 14(12):3057–3071
del Pozo JC, Diaz-Trivino S, Cisneros N, Gutierrez C (2006) The balance between cell division and endoreplication depends on E2FC-DPB, transcription factors regulated by the ubiquitin-SCFSKP2A pathway in Arabidopsis. Plant Cell 18(9):2224–2235. doi:10.1105/tpc.105.039651
Delarue M, Prinsen E, Van Onckelen H, Caboche M, Bellini C (1998) Sur2 mutations of Arabidopsis thaliana define a new locus involved in the control of auxin homeostasis. Plant J 14(5):603–611
Dhondt S, Coppens F, De Winter F, Swarup K, Merks RMH, Inzé D, Bennett MJ, Beemster GTS (2010) SHORT-ROOT and SCARECROW regulate leaf growth in Arabidopsis by stimulating S-phase progression of the cell cycle. Plant Physiol 154(3):1183–1195. doi:10.1104/pp.110.158857
DiDonato RJ, Arbuckle E, Buker S, Sheets J, Tobar J, Totong R, Grisafi P, Fink GR, Celenza JL (2004) Arabidopsis ALF4 encodes a nuclear-localized protein required for lateral root formation. Plant J 37(3):340–353. doi:10.1046/j.1365-313X.2003.01964.x
Doerner P, Jørgensen J-E, You R, Steppuhn J, Lamb C (1996) Control of root growth and development by cyclin expression. Nature 380(6574):520–523. doi:10.1038/380520a0
Donner TJ, Sherr I, Scarpella E (2009) Regulation of preprocambial cell state acquisition by auxin signaling in Arabidopsis leaves. Development 136(19):3235–3246. doi:10.1242/dev.037028
Dubrovsky JG, Doerner PW, Colón-Carmona A, Rost TL (2000) Pericycle cell proliferation and lateral root initiation in Arabidopsis. Plant Physiol 124(4):1648–1657
Dubrovsky JG, Sauer M, Napsucialy-Mendivil S, Ivanchenko MG, Friml J, Shishkova S, Celenza J, Benková E (2008) Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc Natl Acad Sci U S A 105(25):8790–8794. doi:10.1073/pnas.0712307105
Dudits D, Ábrahám E, Miskolczi P, Ayaydin F, Bilgin M, Horváth GV (2011) Cell-cycle control as a target for calcium, hormonal and developmental signals: the role of phosphorylation in the retinoblastoma-centred pathway. Ann Bot 107(7):1193–1202. doi:10.1093/aob/mcr038
Fan M, Xu C, Xu K, Hu Y (2012) LATERAL ORGAN BOUNDARIES DOMAIN transcription factors direct callus formation in Arabidopsis regeneration. Cell Res 22(7):1169–1180. doi:10.1038/cr.2012.63
Feng Z, Sun X, Wang G, Liu H, Zhu J (2012) LBD29 regulates the cell cycle progression in response to auxin during lateral root formation in Arabidopsis thaliana. Ann Bot 110(1):1–10. doi:10.1093/aob/mcs019
Ferreira P, Hemerly A, de Almeida Engler J, Bergounioux C, Burssens S, Van Montagu M, Engler G, Inzé D (1994a) Three discrete classes of Arabidopsis cyclins are expressed during different intervals of the cell cycle. Proc Natl Acad Sci U S A 91(24):11313–11317
Ferreira PCG, Hemerly AS, de Almeida Engler J, Van Montagu M, Engler G, Inzé D (1994b) Developmental expression of the Arabidopsis cyclin gene cyc1At. Plant Cell 6(12):1763–1774
Fukaki H, Tameda S, Masuda H, Tasaka M (2002) Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J 29(2):153–168
Fukaki H, Nakao Y, Okushima Y, Theologis A, Tasaka M (2005) Tissue-specific expression of stabilized SOLITARY-ROOT/IAA14 alters lateral root development in Arabidopsis. Plant J 44(3):382–395. doi:10.1111/j.1365-313X.2005.02537.x
Fukaki H, Taniguchi N, Tasaka M (2006) PICKLE is required for SOLITARY-ROOT/IAA14-mediated repression of ARF7 and ARF19 activity during Arabidopsis lateral root initiation. Plant J 48(3):380–389. doi:10.1111/j.1365-313X.2006.02882.x
Galinha C, Hofhuis H, Luijten M, Willemsen V, Blilou I, Heidstra R, Scheres B (2007) PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature 449(7165):1053–1057. doi:10.1038/nature06206
Gheysen G, Mitchum MG (2011) How nematodes manipulate plant development pathways for infection. Curr Opin Plant Biol 14(4):415–421. doi:10.1016/j.pbi.2011.03.012
Goh T, Joi S, Mimura T, Fukaki H (2012) The establishment of asymmetry in Arabidopsis lateral root founder cells is regulated by LBD16/ASL18 and related LBD/ASL proteins. Development 139(5):883–893. doi:10.1242/dev.071928
Gordon SP, Heisler MG, Reddy GV, Ohno C, Das P, Meyerowitz EM (2007) Pattern formation during de novo assembly of the Arabidopsis shoot meristem. Development 134(19):3539–3548. doi:10.1242/dev.010298
Goverse A, de Almeida Engler J, Verhees J, van der Krol S, Helder J, Gheysen G (2000) Cell cycle activation by plant parasitic nematodes. Plant Mol Biol 43(5–6):747–761
Grieneisen VA, Xu J, Marée AFM, Hogeweg P, Scheres B (2007) Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature 449(7165):1008–1013. doi:10.1038/nature06215
Grunewald W, Cannoot B, Friml J, Gheysen G (2009a) Parasitic nematodes modulate PIN-mediated auxin transport to facilitate infection. PLoS Pathog 5(1):e1000266. doi:10.1371/journal.ppat.1000266
Grunewald W, van Noorden G, Van Isterdael G, Beeckman T, Gheysen G, Mathesius U (2009b) Manipulation of auxin transport in plant roots during Rhizobium symbiosis and nematode parasitism. Plant Cell 21(9):2553–2562. doi:10.1105/tpc.109.069617
Hemerly AS, Ferreira P, de Almeida Engler J, Van Montagu M, Engler G, Inzé D (1993) cdc2a expression in Arabidopsis is linked with competence for cell division. Plant Cell 5(12):1711–1723. doi:10.1105/tpc.5.12.1711
Hewezi T, Baum TJ (2013) Manipulation of plant cells by cyst and root-knot nematode effectors. Mol Plant Microbe Interact 26(1):9–16. doi:10.1094/MPMI-05-12-0106-FI
Heyman J, De Veylder L (2012) The anaphase-promoting complex/cyclosome in control of plant development. Mol Plant 5(6):1182–1194. doi:10.1093/mp/sss094
Himanen K, Boucheron E, Vanneste S, de Almeida Engler J, Inzé D, Beeckman T (2002) Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell 14(10):2339–2351. doi:10.1105/tpc.004960
Himanen K, Vuylsteke M, Vanneste S, Vercruysse S, Boucheron E, Alard P, Chriqui D, Van Montagu M, Inzé D, Beeckman T (2004) Transcript profiling of early lateral root initiation. Proc Natl Acad Sci U S A 101(14):5146–5151. doi:10.1073/pnas.0308702101
Inzé D, De Veylder L (2006) Cell cycle regulation in plant development. Annu Rev Genet 40:77–105. doi:10.1146/annurev.genet.40.110405.090431
Ishida T, Adachi S, Yoshimura M, Shimizu K, Umeda M, Sugimoto K (2010) Auxin modulates the transition from the mitotic cycle to the endocycle in Arabidopsis. Development 137(1):63–71. doi:10.1242/dev.035840
Ito M (2000) Factors controlling cyclin B expression. Plant Mol Biol 43(5–6):677–690
Jansen L, Hollunder J, Roberts I, Forestan C, Fonteyne P, Van Quickenborne C, Zhen R-G, McKersie B, Parizot B, Beeckman T (2013a) Comparative transcriptomics as a tool for the identification of root branching genes in maize. Plant Biotechnol J. doi:10.1111/pbi.12104
Jansen L, Parizot B, Beeckman T (2013b) Inducible system for lateral roots in Arabidopsis thaliana and maize. Methods Mol Biol 959:149–158. doi:10.1007/978-1-62703-221-6_9
John PCL (2007) Hormonal regulation of cell cycle progression and its role in development. In: Inzé D (ed) Cell cycle control and plant development. Blackwell, Oxford, pp 311–334
John PCL, Zhang K, Dong C, Diederich L, Wightman F (1993) p34cdc2 related proteins in control of cell cycle progression, the switch between division and differentiation in tissue development, and stimulation of division by auxin and cytokinin. Aust J Plant Physiol 20(5):503–526
Jurado S, Abraham Z, Manzano C, López-Torrejón G, Pacios LF, Del Pozo JC (2010) The Arabidopsis cell cycle F-box protein SKP2A binds to auxin. Plant Cell 22(12):3891–3904. doi:10.1105/tpc.110.078972
King JJ, Stimart DP, Fisher RH, Bleecker AB (1995) A mutation altering auxin homeostasis and plant morphology in Arabidopsis. Plant Cell 7(12):2023–2037. doi:10.1105/tpc.7.12.2023
Landrieu I, da Costa M, De Veylder L, Dewitte F, Vandepoele K, Hassan S, Wieruszeski J-M, Corellou F, Faure J-D, Van Montagu M, Inzé D, Lippens G (2004) A small CDC25 dual-specificity tyrosine-phosphatase isoform in Arabidopsis thaliana. Proc Natl Acad Sci U S A 101(36):13380–13385. doi:10.1073/pnas.0405248101
Laskowski MJ, Williams ME, Nusbaum HC, Sussex IM (1995) Formation of lateral root meristems is a two-stage process. Development 121(10):3303–3310
Lee HW, Kim NY, Lee DJ, Kim J (2009) LBD18/ASL20 regulates lateral root formation in combination with LBD16/ASL18 downstream of ARF7 and ARF19 in Arabidopsis. Plant Physiol 151(3):1377–1389. doi:10.1104/pp.109.143685
Liscum E, Hangarter RP (1991) Manipulation of ploidy level in cultured haploid Petunia tissue by phytohormone treatments. J Plant Physiol 138(1):33–38
Lloret PG, Casero PJ, Pulgarín A, Navascués J (1989) The behaviour of two cell populations in the pericycle of Allium cepa, Pisum sativum, and Daucus carota during early lateral root development. Ann Bot 63:465–475
Magyar Z, De Veylder L, Atanassova A, Bakó L, Inzé D, Bögre L (2005) The role of the Arabidopsis E2FB transcription factor in regulating auxin-dependent cell division. Plant Cell 17(9):2527–2541. doi:10.1105/tpc.105.033761
Malamy JE, Benfey PN (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124(1):33–44
Manzano C, Ramirez-Parra E, Casimiro I, Otero S, Desvoyes B, De Rybel B, Beeckman T, Casero P, Gutierrez C, del Pozo JC (2012) Auxin and epigenetic regulation of SKP2B, an F-box that represses lateral root formation. Plant Physiol 160(2):749–762. doi:10.1104/pp.112.198341
Martinez MC, Jørgensen J-E, Lawton MA, Lamb CJ, Doerner PW (1992) Spatial pattern of cdc2 expression in relation to meristem activity and cell proliferation during plant development. Proc Natl Acad Sci U S A 89(16):7360–7364
Menges M, de Jager SM, Gruissem W, Murray JAH (2005) Global analysis of the core cell cycle regulators of Arabidopsis identifies novel genes, reveals multiple and highly specific profiles of expression and provides a coherent model for plant cell cycle control. Plant J 41(4):546–566. doi:10.1111/j.1365-313X.2004.02319.x
Monshausen GB (2012) Visualizing Ca2+ signatures in plants. Curr Opin Plant Biol 15(6):677–682. doi:10.1016/j.pbi.2012.09.014
Monshausen GB, Miller ND, Murphy AS, Gilroy S (2011) Dynamics of auxin-dependent Ca2+ and pH signaling in root growth revealed by integrating high-resolution imaging with automated computer vision-based analysis. Plant J 65(2):309–318. doi:10.1111/j.1365-313X.2010.04423.x
Moreno-Risueno MA, Van Norman JM, Moreno A, Zhang J, Ahnert SE, Benfey PN (2010) Oscillating gene expression determines competence for periodic Arabidopsis root branching. Science 329(5997):1306–1311. doi:10.1126/science.1191937
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with Tobacco tissue cultures. Physiol Plant 15:473–497
Murray JAH, Freeman D, Greenwood J, Huntley R, Makkerh J, Riou-Khamlichi C, Sorrell DA, Cockcroft C, Carmichael JP, Soni R, Shah ZH (1998) Plant D cyclins and retinoblastoma protein homologues. In: Francis D, Dudits D, Inzé D (eds) Plant cell division. Portland Press, London, pp 99–127
Niebel A, de Almeida Engler J, Hemerly A, Ferreira P, Inzé D, Van Montagu M, Gheysen G (1996) Induction of cdc2a and cyc1At expression in Arabidopsis thaliana during early phases of nematode-induced feeding cell formation. Plant J 10(6):1037–1043
Okushima Y, Overvoorde PJ, Arima K, Alonso JM, Chan A, Chang C, Ecker JR, Hughes B, Lui A, Nguyen D, Onodera C, Quach H, Smith A, Yu G, Theologis A (2005) Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: unique and overlapping functions of ARF7 and ARF19. Plant Cell 17(2):444–463. doi:10.1105/tpc.104.028316
Okushima Y, Fukaki H, Onoda M, Theologis A, Tasaka M (2007) ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 19(1):118–130. doi:10.1105/tpc.106.047761
Orchard CB, Siciliano I, Sorrell DA, Marchbank A, Rogers HJ, Francis D, Herbert RJ, Suchomelova P, Lipavska H, Azmi A, Van Onckelen H (2005) Tobacco BY-2 cells expressing fission yeast cdc25 bypass a G2/M block on the cell cycle. Plant J 44(2):290–299. doi:10.1111/j.1365-313X.2005.02524.x
Peres A, Churchman ML, Hariharan S, Himanen K, Verkest A, Vandepoele K, Magyar Z, Hatzfeld Y, Van Der Schueren E, Beemster GTS, Frankard V, Larkin JC, Inzé D, De Veylder L (2007) Novel plant-specific cyclin-dependent kinase inhibitors induced by biotic and abiotic stresses. J Biol Chem 282(35):25588–25596. doi:10.1074/jbc.M703326200
Pettkó-Szandtner A, Mészáros T, Horváth GV, Bakó L, Csordás-Tóth É, Blastyák A, Zhiponova M, Miskolczi P, Dudits D (2006) Activation of an alfalfa cyclin-dependent kinase inhibitor by calmodulin-like domain protein kinase. Plant J 46(1):111–123. doi:10.1111/j.1365-313X.2006.02677.x
Ren H, Santner A, del Pozo JC, Murray JAH, Estelle M (2008) Degradation of the cyclin-dependent kinase inhibitor KRP1 is regulated by two different ubiquitin E3 ligases. Plant J 53(5):705–716. doi:10.1111/j.1365-313X.2007.03370.x
Richard C, Lescot M, Inzé D, De Veylder L (2002) Effect of auxin, cytokinin, and sucrose on cell cycle gene expression in Arabidopsis thaliana cell suspension cultures. Plant Cell Tissue Organ Cult 69(2):167–176. doi:10.1023/A:1015241709145
Roudier F, Fedorova E, Lebris M, Lecomte P, Györgyey J, Vaubert D, Horvath G, Abad P, Kondorosi A, Kondorosi E (2003) The Medicago species A2-type cyclin is auxin regulated and involved in meristem formation but dispensable for endoreduplication-associated developmental programs. Plant Physiol 131(3):1091–1103. doi:10.1104/pp.102.011122
Sabelli PA, Nguyen H, Larkins BA (2007) Cell cycle and endosperm development. In: Inzé D (ed) Cell cycle control and plant development, vol 32, Annual plant reviews. Blackwell, Oxford, UK
Sanz L, Dewitte W, Forzani C, Patell F, Nieuwland J, Wen B, Quelhas P, De Jager S, Titmus C, Campilho A, Ren H, Estelle M, Wang H, Murray JAH (2011) The Arabidopsis D-type cyclin CYCD2;1 and the inhibitor ICK2/KRP2 modulate auxin-induced lateral root formation. Plant Cell 23(2):641–660. doi:10.1105/tpc.110.080002
Shishova M, Lindberg S (2010) A new perspective on auxin perception. J Plant Physiol 167(6):417–422. doi:10.1016/j.jplph.2009.12.014
Soni R, Carmichael JP, Shah ZH, Murray JAH (1995) A family of cyclin D homologs from plants differentially controlled by growth regulators and containing the conserved retinoblastoma protein interaction motif. Plant Cell 7(1):85–103
Sorrell DA, Marchbank A, McMahon K, Dickinson JR, Rogers HJ, Francis D (2002) A WEE1 homologue from Arabidopsis thaliana. Planta 215(3):518–522. doi:10.1007/s00425-002-0815-4
Sozzani R, Cui H, Moreno-Risueno MA, Busch W, Van Norman JM, Vernoux T, Brady SM, Dewitte W, Murray JAH, Benfey PN (2010) Spatiotemporal regulation of cell-cycle genes by SHORTROOT links patterning and growth. Nature 466(7302):128–132. doi:10.1038/nature09143
Sugimoto K, Jiao Y, Meyerowitz EM (2010) Arabidopsis regeneration from multiple tissues occurs via a root development pathway. Dev Cell 18(3):463–471. doi:10.1016/j.devcel.2010.02.004
Sun Y, Dilkes BP, Zhang C, Dante RA, Carneiro NP, Lowe KS, Jung R, Gordon-Kamm WJ, Larkins BA (1999) Characterization of maize (Zea mays L.) Wee1 and its activity in developing endosperm. Proc Natl Acad Sci U S A 96(7):4180–4185
Takanashi K, Sugiyama A, Yazaki K (2011) Involvement of auxin distribution in root nodule development of Lotus japonicus. Planta 234(1):73–81. doi:10.1007/s00425-011-1385-0
Tao W, Verbelen J-P (1996) Switching on and off cell division and cell expansion in cultured mesophyll protoplasts of tobacco. Plant Sci 116:107–115
Tardieu F, Granier C (2000) Quantitative analysis of cell division in leaves: methods, developmental patterns and effects of environmental conditions. Plant Mol Biol 43(5–6):555–567
Torrey JG (1950) The induction of lateral roots by indoleacetic acid and root decapitation. Am J Bot 37:257–264
Ulmasov T, Liu Z-B, Hagen G, Guilfoyle TJ (1995) Composite structure of auxin response elements. Plant Cell 7(10):1611–1623. doi:10.1105/tpc.7.10.1611
Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9(11):1963–1971. doi:10.1105/tpc.9.11.1963
Ulmasov T, Hagen G, Guilfoyle TJ (1999) Dimerization and DNA binding of auxin response factors. Plant J 19(3):309–319
Umeda M, Shimotohno A, Yamaguchi M (2005) Control of cell division and transcription by cyclin-dependent kinase-activating kinases in plants. Plant Cell Physiol 46(9):1437–1442. doi:10.1093/pcp/pci170
Valente P, Tao W, Verbelen J-P (1998) Auxins and cytokinins control DNA endoreduplication and deduplication in single cells of tobacco. Plant Sci 134:207–215
Van Bel M, Proost S, Wischnitzki E, Movahedi S, Scheerlinck C, Van de Peer Y, Vandepoele K (2012) Dissecting plant genomes with the PLAZA comparative genomics platform. Plant Physiol 158(2):590–600. doi:10.1104/pp.111.189514
Van Leene J, Hollunder J, Eeckhout D, Persiau G, Van De Slijke E, Stals H, Van Isterdael G, Verkest A, Neirynck S, Buffel Y, De Bodt S, Maere S, Laukens K, Pharazyn A, Ferreira PCG, Eloy N, Renne C, Meyer C, Faure J-D, Steinbrenner J, Beynon J, Larkin JC, Van de Peer Y, Hilson P, Kuiper M, De Veylder L, Van Onckelen H, Inzé D, Witters E, De Jaeger G (2010) Targeted interactomics reveals a complex core cell cycle machinery in Arabidopsis thaliana. Mol Syst Biol 6:397. doi:10.1038/msb.2010.53
van Noorden GE, Kerim T, Goffard N, Wiblin R, Pellerone FI, Rolfe BG, Mathesius U (2007) Overlap of proteome changes in Medicago truncatula in response to auxin and Sinorhizobium meliloti. Plant Physiol 144(2):1115–1131. doi:10.1104/pp.107.099978
Van Norman JM, Xuan W, Beeckman T, Benfey PN (2013) To branch or not to branch: the role of pre-patterning in lateral root formation. Development 140(21):4301–4310. doi:10.1242/dev.090548
Van’t Hof J (1985) Control points within the cell cycle. In: Bryant JA, Francis D (eds) The cell division cycle in plants. Cambridge University Press, Cambridge, pp 1–13
Vandepoele K, Raes J, De Veylder L, Rouzé P, Rombauts S, Inzé D (2002) Genome-wide analysis of core cell cycle genes in Arabidopsis. Plant Cell 14(4):903–916
Vanneste S, De Rybel B, Beemster GTS, Ljung K, De Smet I, Van Isterdael G, Naudts M, Iida R, Gruissem W, Tasaka M, Inzé D, Fukaki H, Beeckman T (2005) Cell cycle progression in the pericycle is not sufficient for SOLITARY ROOT/IAA14-mediated lateral root initiation in Arabidopsis thaliana. Plant Cell 17(11):3035–3050. doi:10.1105/tpc.105.035493
Vieira P, Escudero C, Rodiuc N, Boruc J, Russinova E, Glab N, Mota M, De Veylder L, Abad P, Engler G, de Almeida Engler J (2013) Ectopic expression of Kip-related proteins restrains root-knot nematode-feeding site expansion. New Phytol 199(2):505–519. doi:10.1111/nph.12255
Vodermaier HC (2004) APC/C and SCF: controlling each other and the cell cycle. Curr Biol 14(18):R787–R796. doi:10.1016/j.cub.2004.09.020
Walcher CL, Nemhauser JL (2012) Bipartite promoter element required for auxin response. Plant Physiol 158(1):273–282. doi:10.1104/pp.111.187559
Weimer AK, Nowack MK, Bouyer D, Zhao X, Harashima H, Naseer S, De Winter F, Dissmeyer N, Geldner N, Schnittger A (2012) Retinoblastoma related1 regulates asymmetric cell divisions in Arabidopsis. Plant Cell 24(10):4083–4095. doi:10.1105/tpc.112.104620
Wilmoth JC, Wang S, Tiwari SB, Joshi AD, Hagen G, Guilfoyle TJ, Alonso JM, Ecker JR, Reed JW (2005) NPH4/ARF7 and ARF19 promote leaf expansion and auxin-induced lateral root formation. Plant J 43(1):118–130. doi:10.1111/j.1365-313X.2005.02432.x
Xu K, Liu J, Fan M, Xin W, Hu Y, Xu C (2012) A genome-wide transcriptome profiling reveals the early molecular events during callus initiation in Arabidopsis multiple organs. Genomics 100(2):116–124. doi:10.1016/j.ygeno.2012.05.013
Zhang K, Letham DS, John PCL (1996) Cytokinin controls the cell cycle at mitosis by stimulating the tyrosine dephosphorylation and activation of p34cdc2-like H1 histone kinase. Planta 200(1):2–12
Zhang K, Diederich L, John PCL (2005) The cytokinin requirement for cell division in cultured Nicotiana plumbaginifolia cells can be satisfied by yeast Cdc25 protein tyrosine phosphatase. Implications for mechanisms of cytokinin response and plant development. Plant Physiol 137(1):308–316. doi:10.1104/pp.104.051938
Acknowledgments
M.J.F.D. is indebted to the Agency for Innovation by Science and Technology in Flanders (IWT) for a predoctoral grant. Work in the lab of T.B. is supported by the Interuniversity Attraction Poles Programme IAP7/29 from the Belgian Federal Science Policy Office.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Wien
About this chapter
Cite this chapter
Demeulenaere, M.J.F., Beeckman, T. (2014). The Interplay Between Auxin and the Cell Cycle During Plant Development. In: Zažímalová, E., Petrášek, J., Benková, E. (eds) Auxin and Its Role in Plant Development. Springer, Vienna. https://doi.org/10.1007/978-3-7091-1526-8_7
Download citation
DOI: https://doi.org/10.1007/978-3-7091-1526-8_7
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-1525-1
Online ISBN: 978-3-7091-1526-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)