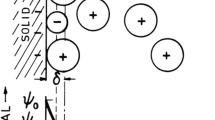
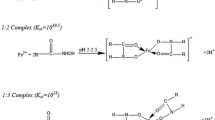
Abstract
Montana Tech has been engaged in fundamental research on the processing of critical materials for more than a decade. Efforts include examining novel reagents for the flotation of Rare Earth Minerals (REMs). For this paper, research results on a collector, salicyl hydroxamic acid (SHA), are presented. Various REMs were examined and include the rare earth oxides (REOs), carbonates (RECs), and phosphates (REPs) of a suite of Rare Earth Elements (REEs). Differences are attributed to solution and surface chemistry, coordination number, and ionic diameter. SHA adsorption follows an ion-exchange process that leads to chemisorbed and surface-precipitated states, depending mostly on pH. Many effects are directly attributed to lanthanide contraction. Results should also be applicable to other REM/collector systems and further suggest that REM flotation should consider dual collectors.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Alonso E, Sherman AM, Wallington TJ, Everson MP, Field FR, Roth R, Kirchain RE (2012) Evaluating rare earth availability: a case with revolutionary demand from clean
Haxel GB, Hedrick JB, Oris GJ (2005) Rare earth elements-critical resources for high technology. Retrieved from U.S. Geological Survey. https://pubs.usgs.gov/fs/2002/fs087-02. Accessed 17 May 2005
Long KR, Van Gosen BS, Foley NK, Cordier D (2010) The principal rare earth elements deposits of the United States—a summary of domestic deposits and a global perspective. Retrieved from U.S. Geological Survey Scientific Investigations Report 2010-5220: https://pubs.usgs.gov/sir/2010/5220/
Fuerstenau PDW (1983) The adsorption of Hydroxamate on semi-soluble minerals, part I: adsorption on barite, calcite, and bastnasite. Colloids Surf 8:103–119
Kawabe I (1992) Lanthanide terad effect in the Ln3+ ionic radii and refined spin-pairing energy theory. Geochem J 26: 309–335
Shannon R (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides
Grupta C, Krishnamurthy N (2005) Extractive metallurgy of rare earths. CRC Press, Boca Raton
Young C, Downey J, Gleason B (2017) Task 004—recovery of rare earth elements with advanced processing technologies, Aberdeen, MD, USA
Anderson CD (2015) Improved understanding of rare earth surface chemistry and its application to froth flotation. Colorado School of Mines, Golden
Huang HH (2018) StabCal: software for thermodynamic stability calculations. Montana Tech of The University of Montana, Butte, MT, USA
Jun R, Wenmei W, Jiake L, Gaoyun Z, Fangqiong T (2003) Progress of flotation reagents of rare earth minerals in China. J Rare Earths 21(1):1–8
Chatterjee B (1978) Donor properties of hydroxamic acids. Coordination Chem Rev 26(3):281–303
Natarajan R, Fuerstenau DW (1983) Adsorption and flotation behavior of manganese dioxide in the presence of octyl hydroxamate. Int J Miner Process, 139–153
Galt G (2017) Thesis: adsorption of salicylhydroxamic acid on selected rare earth oxides and carbonates. Montana Tech of The University of Montana, Butte, MT, USA
Fuerstenau PDW (1991) The role of inorganic and organic reagents in the flotation of rare-earth ores. Int J Miner Process
Zhang W, Honaker R (2017) Adsorption of octanohydroxamic acid on monazite surfaces. University of Kentucky, Department of Mining Engineering, Lexington
Young C, Miller JD (1993) Surface phase transitions of adsorbed collector molecules as revealed by Insitu FT-IR/IRS spectroscopy
Xia L, Hart B, Chelgani S, Douglas K (2014) Hydroxamate collectors for rare earth minerals flotation. In: Conference of metallurgists proceedings. Canadian Institute of Mining, Metallurgy and Petroleum, Vancouver, Canada
LaDouceur R, Young C (2015) Modeling and optimization of rare earth flotation. In: SME annual conference and expo, Denver, CO, USA
Acknowledgements
Thanks are extended to Marc ‘Freddy’ Sime for his coordinated research contributions and discussions and to ARL for their funding of this critical materials research.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
Research was sponsored by the Army Research Laboratory and was accomplished under Cooperative Agreement Number W911NF-15-2-0020. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the Army Research Laboratory or the U.S. Government. The U.S. Government is authorized to reproduce and distribute reprints for Government purposes notwithstanding any copyright notation herein.
Rights and permissions
Copyright information
© 2018 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Trant, S., Galt, G., Das, A., Young, C.A. (2018). Fundamental Understanding of the Flotation Chemistry of Rare Earth Minerals. In: Davis, B., et al. Extraction 2018. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-319-95022-8_218
Download citation
DOI: https://doi.org/10.1007/978-3-319-95022-8_218
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-95021-1
Online ISBN: 978-3-319-95022-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)