Abstract
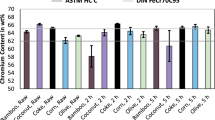
The production of ferrochrome via carbothermic reduction in submerged arc furnaces is an energy-intensive process relying on the usage of coal and coke as reducing agents. The pre-reduction of chromite in a rotary kiln is currently carried out to decrease the specific electric energy consumption in the smelting furnace. However, as fossil reductants are still needed for reduction, CO2 is emitted. The usage of bio-based carbon with a faster carbon cycle compared to fossil reductants could be an option to decrease the specific CO2 footprint of Ferrochrome production. In this paper, the pre-reduction of chromite was investigated using various bio-based reducing agents and lignite coke as a fossil reference. Isothermal reduction trials were conducted at 1000, 1150, and 1300 °C and different holding times. While at lower temperatures the pre-reduction was insufficient, the bio-based reducing agents yield a degree of reaction between 61.0% and 65.4% at 1300 °C reaction times of 360 min. The highest degree of reaction is reached using coconut charcoal, followed by corn, olive, and bamboo charcoal. Coke results in the lowest degree of reaction with 51.9%. While the bio-based reducing agents performed similar after long reaction times, significant deviations were observed for shorter reaction times. X-ray diffraction was carried out to investigate the obtained product, which showed that the pre-reduction was mostly due to the formation of carbides, while the intensity of metals in the sample was rather low.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Sommerfeld M, Friedrich B (2021) Replacing fossil carbon in the production of ferroalloys with a focus on bio-based carbon: a review. Minerals 11(11):1286. https://doi.org/10.3390/min11111286
Fick G, Mirgaux O, Neau P, Patisson F (2014) Using biomass for pig iron production: a technical, environmental and economical assessment. Waste Biomass Valorization 5(1):43–55. https://doi.org/10.1007/s12649-013-9223-1
Suopajärvi H, Umeki K, Mousa E, Hedayati A, Romar H, Kemppainen A, Wang C, Phounglamcheik A, Tuomikoski S, Norberg N, Andefors A, Öhman M, Lassi U, Fabritius T (2018) Use of biomass in integrated steelmaking—status quo, future needs and comparison to other low-CO2 steel production technologies. Appl Energy 213:384–407. https://doi.org/10.1016/j.apenergy.2018.01.060
Mousa E, Wang C, Riesbeck J, Larsson M (2016) Biomass applications in iron and steel industry: an overview of challenges and opportunities. Renew Sustain Energy Rev 65:1247–1266. https://doi.org/10.1016/j.rser.2016.07.061
Quader MA, Ahmed S, Ghazilla RAR, Ahmed S, Dahari M (2015) A comprehensive review on energy efficient CO2 breakthrough technologies for sustainable green iron and steel manufacturing. Renew Sustain Energy Rev 50:594–614. https://doi.org/10.1016/J.RSER.2015.05.026
Suopajärvi H, Kemppainen A, Haapakangas J, Fabritius T (2017) Extensive review of the opportunities to use biomass-based fuels in iron and steelmaking processes. J Clean Prod 148:709–734. https://doi.org/10.1016/j.jclepro.2017.02.029
Rosenfeld DC, Böhm H, Lindorfer J, Lehner M (2020) Scenario analysis of implementing a power-to-gas and biomass gasification system in an integrated steel plant: a techno-economic and environmental study. Renew Energy 147:1511–1524. https://doi.org/10.1016/J.RENENE.2019.09.053
Mandova H, Leduc S, Wang C, Wetterlund E, Patrizio P, Gale W, Kraxner F (2018) Possibilities for CO2 emission reduction using biomass in European integrated steel plants. Biomass Bioenerg 115:231–243. https://doi.org/10.1016/j.biombioe.2018.04.021
Suopajärvi H, Pongráczb E, Fabritiusa T (2013) The potential of using biomass-based reducing agents in the blast furnace: a review of thermochemical conversion technologies and assessments related to sustainability. Renew Sustain Energy Rev 25:511–528. https://doi.org/10.1016/j.rser.2013.05.005
Gupta RC (2003) Woodchar as a sustainable reductant for ironmaking in the 21st century. Min Proc Ext Met Rev 24(3–4):203–231. https://doi.org/10.1080/714856822
Wei R, Zhang L, Cang D, Li J, Li X, Xu CC (2017) Current status and potential of biomass utilization in ferrous metallurgical industry. Renew Sustain Energy Rev 68:511–524. https://doi.org/10.1016/j.rser.2016.10.013
Basson J, Daavittila J (2013) High carbon ferrochrome technology. In: Gasik M (ed) Handbook of ferroalloys. Elsevier/Butterworth-Heinemann. Amsterdam, The Netherlands, pp 317–363
Kleynhans ELJ, Beukes JP, van Zyl PG, Fick JIJ (2017) Techno-economic feasibility of a pre-oxidation process to enhance prereduction of chromite. J S Afr Inst Min Metall 117(5):457–468. https://doi.org/10.17159/2411-9717/2017/v117n5a8
Kleynhans ELJ, Neizel BW, Beukes JP, van Zyl PG (2016) Utilisation of pre-oxidised ore in the pelletised chromite pre-reduction process. Miner Eng 92:114–124. https://doi.org/10.1016/j.mineng.2016.03.005
Kleynhans ELJ, Beukes JP, van Zyl PG, Bunt JR, Nkosi NSB, Venter M (2017) The effect of carbonaceous reductant selection on chromite pre-reduction. Metall Mater Trans B 48(2):827–840. https://doi.org/10.1007/s11663-016-0878-4
van Staden Y, Beukes JP, van Zyl PG, Ringdalen E, Tangstad M, Kleynhans ELJ, Bunt JR (2018) Damring formation during rotary kiln chromite pre-reduction: effects of pulverized carbonaceous fuel selection and partial pellet melting. Metall Mater Trans B 49(6):3488–3503. https://doi.org/10.1007/s11663-018-1376-7
Mohale GTM, Beukes JP, Kleynhans ELJ, van Zyl PG, Bunt JR, Tiedt LR, Venter AD, Jordaan A (2017) SEM image processing as an alternative method to determine chromite pre-reduction. J S Afr Inst Min Metall 117(11):1045–1052. https://doi.org/10.17159/2411-9717/2017/v117n11a9
Neizel BW, Beukes JP, van Zyl PG, Dawson NF (2013) Why is CaCO3 not used as an additive in the pelletised chromite pre-reduction process? Miner Eng 45:115–120. https://doi.org/10.1016/j.mineng.2013.02.015
Paktunc D, Thibault Y, Sokhanvaran S, Yu D (2018) Influences of alkali fluxes on direct reduction of chromite for ferrochrome production. J S Afr Inst Min Metall 118(12). https://doi.org/10.17159/2411-9717/2018/v118n12a9
Sokhanvaran S, Paktunc D, Barnes A (2018) NaOH-assisted direct reduction of Ring of Fire chromite ores, and the associated implications for processing. J S Afr Inst Min Metall 118(6). https://doi.org/10.17159/2411-9717/2018/v118n6a4
Yu D, Paktunc D (2018) Kinetics and mechanisms of the carbothermic reduction of chromite in the presence of nickel. J Therm Anal Calorim 132(1):143–154. https://doi.org/10.1007/s10973-017-6936-6
Sommerfeld M, Friedrich B (2021) Proposition of a thermogravimetric method to measure the ferrous iron content in metallurgical-grade chromite. Minerals 12(2):109. https://doi.org/10.3390/min12020109
Bale CW, Bélisle E, Chartrand P, Decterov SA, Eriksson G, Gheribi AE, Hack K, Jung I-H, Kang Y-B, Melançon J, Pelton AD, Petersen S, Robelin C, Sangster J, Spencer P, van Ende M-A (2016) FactSage thermochemical software and databases, 2010–2016. Calphad 54:35–53. https://doi.org/10.1016/j.calphad.2016.05.002
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Sommerfeld, M., Friedrich, B. (2022). Toward Green Ferroalloys: Replacement of Fossil Reductants in the Pre-reduction Process of Chromite by Bio-Based Alternatives. In: Lazou, A., Daehn, K., Fleuriault, C., Gökelma, M., Olivetti, E., Meskers, C. (eds) REWAS 2022: Developing Tomorrow’s Technical Cycles (Volume I). The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-030-92563-5_65
Download citation
DOI: https://doi.org/10.1007/978-3-030-92563-5_65
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-92562-8
Online ISBN: 978-3-030-92563-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)