Abstract
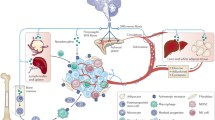
The central and autonomic nervous systems interact and converge to build up an adrenergic nerve network capable of promoting cancer. While a local adrenergic sympathetic innervation in peripheral solid tumors influences cancer and stromal cell behavior, the brain can participate to the development of cancer through an intermixed dysregulation of the sympathoadrenal system, adrenergic neurons, and the hypothalamo-pituitary-adrenal axis. A deeper understanding of the adrenergic nerve circuitry within the brain and tumors and its interactions with the microenvironment should enable elucidation of original mechanisms of cancer and novel therapeutic strategies.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Rich AR (2007) On the frequency of occurrence of occult carcinoma of the prostrate. 1934. Int J Epidemiol 36:274–277. https://doi.org/10.1093/ije/dym050
Sakr WA, Haas GP, Cassin BF, Pontes JE, Crissman JD (1993) The frequency of carcinoma and intraepithelial neoplasia of the prostate in young male patients. J Urol 150:379–385. https://doi.org/10.1016/s0022-5347(17)35487-3
Nielsen M, Thomsen JL, Primdahl S, Dyreborg U, Andersen JA (1987) Breast cancer and atypia among young and middle-aged women: a study of 110 medicolegal autopsies. Br J Cancer 56:814–819. https://doi.org/10.1038/bjc.1987.296
Bissell MJ, Hines WC (2011) Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med 17:320–329. https://doi.org/10.1038/nm.2328
Binnewies M et al (2018) Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 24:541–550. https://doi.org/10.1038/s41591-018-0014-x
Hanahan D, Coussens LM (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21:309–322. https://doi.org/10.1016/j.ccr.2012.02.022
Grothey A, Galanis E (2009) Targeting angiogenesis: progress with anti-VEGF treatment with large molecules. Nat Rev Clin Oncol 6:507–518. https://doi.org/10.1038/nrclinonc.2009.110
Ferrara N et al (1996) Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380:439–442
Paez-Ribes M et al (2009) Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 15:220–231
Ebos JM et al (2009) Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 15:232–239
Van der Veldt AA et al (2012) Rapid decrease in delivery of chemotherapy to tumors after anti-VEGF therapy: implications for scheduling of anti-angiogenic drugs. Cancer Cell 21:82–91. https://doi.org/10.1016/j.ccr.2011.11.023
Conley SJ et al (2012) Antiangiogenic agents increase breast cancer stem cells via the generation of tumor hypoxia. Proc Natl Acad Sci U S A 109:2784–2789. https://doi.org/10.1073/pnas.1018866109
Krummel MF, Allison JP (1995) CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med 182:459–465
Leach DR, Krummel MF, Allison JP (1996) Enhancement of antitumor immunity by CTLA-4 blockade. Science 271:1734–1736
Ishida Y, Agata Y, Shibahara K, Honjo T (1992) Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 11:3887–3895
Dong H, Zhu G, Tamada K, Chen L (1999) B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 5:1365–1369. https://doi.org/10.1038/70932
Freeman GJ et al (2000) Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192:1027–1034
Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T (2013) A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol 14:1212–1218. https://doi.org/10.1038/ni.2762
Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A (2017) Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 168:707–723. https://doi.org/10.1016/j.cell.2017.01.017
Schoenfeld AJ, Hellmann MD (2020) Acquired Resistance to Immune Checkpoint Inhibitors. Cancer Cell 37:443–455. https://doi.org/10.1016/j.ccell.2020.03.017
Marusyk A, Janiszewska M, Polyak K (2020) Intratumor Heterogeneity: The Rosetta Stone of Therapy Resistance. Cancer Cell 37:471–484. https://doi.org/10.1016/j.ccell.2020.03.007
Magnon C et al (2013) Autonomic nerve development contributes to prostate cancer progression. Science 341:1236361. https://doi.org/10.1126/science.1236361
Dobrenis K, Gauthier LR, Barroca V, Magnon C (2015) Granulocyte colony-stimulating factor off-target effect on nerve outgrowth promotes prostate cancer development. Int J Cancer 136:982–988. https://doi.org/10.1002/ijc.29046
Boilly B, Faulkner S, Jobling P, Hondermarck H (2017) Nerve Dependence: From Regeneration to Cancer. Cancer Cell 31:342–354. https://doi.org/10.1016/j.ccell.2017.02.005
Hayakawa Y et al (2017) Nerve Growth Factor Promotes Gastric Tumorigenesis through Aberrant Cholinergic Signaling. Cancer Cell 31:21–34. https://doi.org/10.1016/j.ccell.2016.11.005
Zhao CM et al (2014) Denervation suppresses gastric tumorigenesis. Sci Transl Med 6:250ra115. https://doi.org/10.1126/scitranslmed.3009569
Pundavela J et al (2015) Nerve fibers infiltrate the tumor microenvironment and are associated with nerve growth factor production and lymph node invasion in breast cancer. Mol Oncol 9:1626–1635. https://doi.org/10.1016/j.molonc.2015.05.001
Peterson SC et al (2015) Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell 16:400–412. https://doi.org/10.1016/j.stem.2015.02.006
Stopczynski RE et al (2014) Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer Res 74:1718–1727. https://doi.org/10.1158/0008-5472.CAN-13-2050
Saloman JL et al (2016) Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc Natl Acad Sci U S A 113:3078–3083. https://doi.org/10.1073/pnas.1512603113
Liebl F et al (2013) The severity of neural invasion is associated with shortened survival in colon cancer. Clin Cancer Res 19:50–61. https://doi.org/10.1158/1078-0432.CCR-12-2392
Gibbins I (2013) Functional organization of autonomic neural pathways. Organogenesis 9:169–175. https://doi.org/10.4161/org.25126
Goldstein DS (2010) Adrenal responses to stress. Cell Mol Neurobiol 30:1433–1440. https://doi.org/10.1007/s10571-010-9606-9
Ernsberger U, Rohrer H (2018) Sympathetic tales: subdivisons of the autonomic nervous system and the impact of developmental studies. Neural Dev 13:20. https://doi.org/10.1186/s13064-018-0117-6
Jansen AS, Nguyen XV, Karpitskiy V, Mettenleiter TC, Loewy AD (1995) Central command neurons of the sympathetic nervous system: basis of the fight-or-flight response. Science 270:644–646. https://doi.org/10.1126/science.270.5236.644
Thaker PH et al (2006) Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med 12:939–944. https://doi.org/10.1038/nm1447
Sloan EK et al (2010) The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res 70:7042–7052. https://doi.org/10.1158/0008-5472.CAN-10-0522
Cohen S, Levi-Montalcini R, Hamburger V (1954) A Nerve Growth-Stimulating Factor Isolated from Sarcom as 37 and 180. Proc Natl Acad Sci U S A 40:1014–1018. https://doi.org/10.1073/pnas.40.10.1014
Levi-Montalcini R, Meyer H, Hamburger V (1954) In vitro experiments on the effects of mouse sarcomas 180 and 37 on the spinal and sympathetic ganglia of the chick embryo. Cancer Res 14:49–57
Barnbrook DH (1953) Pheochromocytoma; an alternative surgical approach. Can Med Assoc J 68:245–247
Scharrer B (1949) Gastric cancer experimentally induced in insects by nerve severance. J Natl Cancer Inst 10:375; Disc, 399–403
Batkin S, Piette LH, Wildman E (1970) Effect of muscle denervation on growth of transplanted tumor in mice. Proc Natl Acad Sci U S A 67:1521–1527. https://doi.org/10.1073/pnas.67.3.1521
De Sousa Pereira A (1946) A basis for sympathectomy for cancer of the cervix uteri. Arch Surg 52:260–285. https://doi.org/10.1001/archsurg.1946.01230050265003
Reiche EM, Nunes SO, Morimoto HK (2004) Stress, depression, the immune system, and cancer. Lancet Oncol 5:617–625. https://doi.org/10.1016/S1470-2045(04)01597-9
Riley V (1975) Mouse mammary tumors: alteration of incidence as apparent function of stress. Science 189:465–467. https://doi.org/10.1126/science.168638
Liebig C, Ayala G, Wilks JA, Berger DH, Albo D (2009) Perineural invasion in cancer: a review of the literature. Cancer 115:3379–3391. https://doi.org/10.1002/cncr.24396
Amit M, Na’ara S, Gil Z (2016) Mechanisms of cancer dissemination along nerves. Nat Rev Cancer 16:399–408. https://doi.org/10.1038/nrc.2016.38
Carter RL, Foster CS, Dinsdale EA, Pittam MR (1983) Perineural spread by squamous carcinomas of the head and neck: a morphological study using antiaxonal and antimyelin monoclonal antibodies. J Clin Pathol 36:269–275. https://doi.org/10.1136/jcp.36.3.269
Soo KC et al (1986) Prognostic implications of perineural spread in squamous carcinomas of the head and neck. Laryngoscope 96:1145–1148. https://doi.org/10.1288/00005537-198610000-00015
Anderson PR, Hanlon AL, Patchefsky A, Al-Saleem T, Hanks GE (1998) Perineural invasion and Gleason 7-10 tumors predict increased failure in prostate cancer patients with pretreatment PSA <10 ng/ml treated with conformal external beam radiation therapy. Int J Radiat Oncol Biol Phys 41:1087–1092
Ayala GE et al (2001) In vitro dorsal root ganglia and human prostate cell line interaction: redefining perineural invasion in prostate cancer. Prostate 49:213–223. https://doi.org/10.1002/pros.1137
Isaacs J, Cancer T (2013) Prostate cancer takes nerve. Science 341:134–135. https://doi.org/10.1126/science.1241776
Ayala GE et al (2008) Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res 14:7593–7603
Cryer PE (1980) Physiology and pathophysiology of the human sympathoadrenal neuroendocrine system. N Engl J Med 303:436–444. https://doi.org/10.1056/NEJM198008213030806
Kvetnansky R et al (1995) Sympathoadrenal system in stress. Interaction with the hypothalamic-pituitary-adrenocortical system. Ann N Y Acad Sci 771:131–158. https://doi.org/10.1111/j.1749-6632.1995.tb44676.x
Ehrhart-Bornstein M, Bornstein SR (2008) Cross-talk between adrenal medulla and adrenal cortex in stress. Ann N Y Acad Sci 1148:112–117. https://doi.org/10.1196/annals.1410.053
Zuckerman-Levin N, Tiosano D, Eisenhofer G, Bornstein S, Hochberg Z (2001) The importance of adrenocortical glucocorticoids for adrenomedullary and physiological response to stress: a study in isolated glucocorticoid deficiency. J Clin Endocrinol Metab 86:5920–5924. https://doi.org/10.1210/jcem.86.12.8106
Kvetnansky R et al (2006) Gene expression of phenylethanolamine N-methyltransferase in corticotropin-releasing hormone knockout mice during stress exposure. Cell Mol Neurobiol 26:735–754. https://doi.org/10.1007/s10571-006-9063-7
Yoshida-Hiroi M et al (2002) Chromaffin cell function and structure is impaired in corticotropin-releasing hormone receptor type 1-null mice. Mol Psychiatry 7:967–974. https://doi.org/10.1038/sj.mp.4001143
Herman JP et al (2016) Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr Physiol 6:603–621. https://doi.org/10.1002/cphy.c150015
Brown MR, Fisher LA (1986) Glucocorticoid suppression of the sympathetic nervous system and adrenal medulla. Life Sci 39:1003–1012. https://doi.org/10.1016/0024-3205(86)90289-4
Munck A, Guyre PM, Holbrook NJ (1984) Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev 5:25–44. https://doi.org/10.1210/edrv-5-1-25
Munck A, Naray-Fejes-Toth A (1992) The ups and downs of glucocorticoid physiology. Permissive and suppressive effects revisited. Mol Cell Endocrinol 90:C1–C4. https://doi.org/10.1016/0303-7207(92)90091-j
Hassan S et al (2013) Behavioral stress accelerates prostate cancer development in mice. J Clin Invest 123:874–886. https://doi.org/10.1172/JCI63324
Madden KS, Szpunar MJ, Brown EB (2011) beta-Adrenergic receptors (beta-AR) regulate VEGF and IL-6 production by divergent pathways in high beta-AR-expressing breast cancer cell lines. Breast Cancer Res Treat 130:747–758. https://doi.org/10.1007/s10549-011-1348-y
Eng JW et al (2015) Housing temperature-induced stress drives therapeutic resistance in murine tumour models through beta2-adrenergic receptor activation. Nat Commun 6:6426. https://doi.org/10.1038/ncomms7426
Kim-Fuchs C et al (2014) Chronic stress accelerates pancreatic cancer growth and invasion: a critical role for beta-adrenergic signaling in the pancreatic microenvironment. Brain Behav Immun 40:40–47. https://doi.org/10.1016/j.bbi.2014.02.019
Hasegawa H, Saiki I (2002) Psychosocial stress augments tumor development through beta-adrenergic activation in mice. Jpn J Cancer Res 93:729–735. https://doi.org/10.1111/j.1349-7006.2002.tb01313.x
Goldfarb Y et al (2011) Improving postoperative immune status and resistance to cancer metastasis: a combined perioperative approach of immunostimulation and prevention of excessive surgical stress responses. Ann Surg 253:798–810. https://doi.org/10.1097/SLA.0b013e318211d7b5
Pasquier E et al (2013) beta-blockers increase response to chemotherapy via direct antitumour and anti-angiogenic mechanisms in neuroblastoma. Br J Cancer 108:2485–2494. https://doi.org/10.1038/bjc.2013.205
Wolter JK et al (2014) Anti-tumor activity of the beta-adrenergic receptor antagonist propranolol in neuroblastoma. Oncotarget 5:161–172. https://doi.org/10.18632/oncotarget.1083
Lutgendorf SK et al (2011) Social isolation is associated with elevated tumor norepinephrine in ovarian carcinoma patients. Brain Behav Immun 25:250–255. https://doi.org/10.1016/j.bbi.2010.10.012
Campbell JP et al (2012) Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS Biol 10:e1001363. https://doi.org/10.1371/journal.pbio.1001363
Buckingham JC (1996) Fifteenth Gaddum Memorial Lecture December 1994. Stress and the neuroendocrine-immune axis: the pivotal role of glucocorticoids and lipocortin 1. Br J Pharmacol 118:1–19. https://doi.org/10.1111/j.1476-5381.1996.tb15360.x
Obradovic MMS et al (2019) Glucocorticoids promote breast cancer metastasis. Nature 567:540–544. https://doi.org/10.1038/s41586-019-1019-4
Mohammadpour H et al (2019) beta2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. J Clin Invest 129:5537–5552. https://doi.org/10.1172/JCI129502
Ayala GE et al (2004) Growth and survival mechanisms associated with perineural invasion in prostate cancer. Cancer Res 64:6082–6090. https://doi.org/10.1158/0008-5472.CAN-04-0838
Kobayashi T, Kihara K, Hyochi N, Masuda H, Sato K (2003) Spontaneous regeneration of the seriously injured sympathetic pathway projecting to the prostate over a long period in the dog. BJU Int 91:868–872
Frisbie JH, Binard J (1994) Low prevalence of prostatic cancer among myelopathy patients. J Am Paraplegia Soc 17:148–149. https://doi.org/10.1080/01952307.1994.11735926
Rutledge A, Jobling P, Walker MM, Denham JW, Hondermarck H (2017) Spinal cord injuries and nerve dependence in prostate cancer. Trends Cancer 3:812–815. https://doi.org/10.1016/j.trecan.2017.10.001
You H et al (2020) Sight and switch off: nerve density visualization for interventions targeting nerves in prostate cancer. Sci Adv 6:eaax6040. https://doi.org/10.1126/sciadv.aax6040
Renz BW et al (2018) beta2 adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell 34:863–867. https://doi.org/10.1016/j.ccell.2018.10.010
Allen JK et al (2018) Sustained adrenergic signaling promotes intratumoral innervation through BDNF induction. Cancer Res 78:3233–3242. https://doi.org/10.1158/0008-5472.CAN-16-1701
Madeo M et al (2018) Cancer exosomes induce tumor innervation. Nat Commun 9:4284. https://doi.org/10.1038/s41467-018-06640-0
Kamiya A et al (2019) Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat Neurosci 22:1289–1305. https://doi.org/10.1038/s41593-019-0430-3
Mauffrey P et al (2019) Progenitors from the central nervous system drive neurogenesis in cancer. Nature 569:672–678. https://doi.org/10.1038/s41586-019-1219-y
Alhayek S, Preuss CV (2020) In StatPearls
Frielle T et al (1987) Cloning of the cDNA for the human beta 1-adrenergic receptor. Proc Natl Acad Sci U S A 84:7920–7924. https://doi.org/10.1073/pnas.84.22.7920
McGraw DW, Liggett SB (2005) Molecular mechanisms of beta2-adrenergic receptor function and regulation. Proc Am Thorac Soc 2:292–296; discussion 311–292. https://doi.org/10.1513/pats.200504-027SR
Kobilka BK et al (1987) cDNA for the human beta 2-adrenergic receptor: a protein with multiple membrane-spanning domains and encoded by a gene whose chromosomal location is shared with that of the receptor for platelet-derived growth factor. Proc Natl Acad Sci U S A 84:46–50. https://doi.org/10.1073/pnas.84.1.46
Emorine LJ et al (1989) Molecular characterization of the human beta 3-adrenergic receptor. Science 245:1118–1121. https://doi.org/10.1126/science.2570461
Ahlquist RP (1948) A study of the adrenotropic receptors. Am J Phys 153:586–600. https://doi.org/10.1152/ajplegacy.1948.153.3.586
Bylund DB (2007) Alpha- and beta-adrenergic receptors: Ahlquist’s landmark hypothesis of a single mediator with two receptors. Am J Physiol Endocrinol Metab 293:E1479–E1481. https://doi.org/10.1152/ajpendo.00664.2007
Sood AK et al (2010) Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. J Clin Invest 120:1515–1523. https://doi.org/10.1172/JCI40802
Watkins JL et al (2015) Clinical impact of selective and nonselective beta-blockers on survival in patients with ovarian cancer. Cancer 121:3444–3451. https://doi.org/10.1002/cncr.29392
Lang K et al (2004) Induction of a metastatogenic tumor cell type by neurotransmitters and its pharmacological inhibition by established drugs. Int J Cancer 112:231–238. https://doi.org/10.1002/ijc.20410
Choy C et al (2016) Inhibition of beta2-adrenergic receptor reduces triple-negative breast cancer brain metastases: The potential benefit of perioperative beta-blockade. Oncol Rep 35:3135–3142. https://doi.org/10.3892/or.2016.4710
Montoya A et al (2017) Use of non-selective beta-blockers is associated with decreased tumor proliferative indices in early stage breast cancer. Oncotarget 8:6446–6460. https://doi.org/10.18632/oncotarget.14119
Sastry KS et al (2007) Epinephrine protects cancer cells from apoptosis via activation of cAMP-dependent protein kinase and BAD phosphorylation. J Biol Chem 282:14094–14100. https://doi.org/10.1074/jbc.M611370200
Guo K et al (2009) Norepinephrine-induced invasion by pancreatic cancer cells is inhibited by propranolol. Oncol Rep 22:825–830. https://doi.org/10.3892/or_00000505
Zhang D, Ma QY (2010) Hu, H. T. & Zhang, M. beta2-adrenergic antagonists suppress pancreatic cancer cell invasion by inhibiting CREB, NFkappaB and AP-1. Cancer Biol Ther 10:19–29. https://doi.org/10.4161/cbt.10.1.11944
Al-Wadei HA et al (2012) Social stress promotes and gamma-aminobutyric acid inhibits tumor growth in mouse models of non-small cell lung cancer. Cancer Prev Res (Phila) 5:189–196. https://doi.org/10.1158/1940-6207.CAPR-11-0177
Park PG, Merryman J, Orloff M, Schuller HM (1995) Beta-adrenergic mitogenic signal transduction in peripheral lung adenocarcinoma: implications for individuals with preexisting chronic lung disease. Cancer Res 55:3504–3508
Wong HP et al (2011) Effects of adrenaline in human colon adenocarcinoma HT-29 cells. Life Sci 88:1108–1112. https://doi.org/10.1016/j.lfs.2011.04.007
Moretti S et al (2013) beta-adrenoceptors are upregulated in human melanoma and their activation releases pro-tumorigenic cytokines and metalloproteases in melanoma cell lines. Lab Investig 93:279–290. https://doi.org/10.1038/labinvest.2012.175
Sardi I et al (2013) Expression of beta-adrenergic receptors in pediatric malignant brain tumors. Oncol Lett 5:221–225. https://doi.org/10.3892/ol.2012.989
Whitsett JA, Burdsall J, Workman L (1983) Hollinger, B. & Neely, J. beta-Adrenergic receptors in pediatric tumors: uncoupled beta 1-adrenergic receptor in Ewing’s sarcoma. J Natl Cancer Inst 71:779–786
Calvani M et al (2015) Norepinephrine promotes tumor microenvironment reactivity through beta3-adrenoreceptors during melanoma progression. Oncotarget 6:4615–4632. https://doi.org/10.18632/oncotarget.2652
Perrone MG, Notarnicola M, Caruso MG, Tutino V, Scilimati A (2008) Upregulation of beta3-adrenergic receptor mRNA in human colon cancer: a preliminary study. Oncology 75:224–229. https://doi.org/10.1159/000163851
Chisholm KM et al (2012) beta-Adrenergic receptor expression in vascular tumors. Mod Pathol 25:1446–1451. https://doi.org/10.1038/modpathol.2012.108
Zhang D et al (2016) Stem cell and neurogenic gene-expression profiles link prostate basal cells to aggressive prostate cancer. Nat Commun 7:10798. https://doi.org/10.1038/ncomms10798
Hara MR, Sachs BD, Caron MG, Lefkowitz RJ (2013) Pharmacological blockade of a beta(2)AR-beta-arrestin-1 signaling cascade prevents the accumulation of DNA damage in a behavioral stress model. Cell Cycle 12:219–224. https://doi.org/10.4161/cc.23368
Hara MR et al (2011) A stress response pathway regulates DNA damage through beta2-adrenoreceptors and beta-arrestin-1. Nature 477:349–353. https://doi.org/10.1038/nature10368
Reeder A et al (2015) Stress hormones reduce the efficacy of paclitaxel in triple negative breast cancer through induction of DNA damage. Br J Cancer 112:1461–1470. https://doi.org/10.1038/bjc.2015.133
Armaiz-Pena GN et al (2013) Src activation by beta-adrenoreceptors is a key switch for tumour metastasis. Nat Commun 4:1403. https://doi.org/10.1038/ncomms2413
Shi M et al (2011) The beta2-adrenergic receptor and Her2 comprise a positive feedback loop in human breast cancer cells. Breast Cancer Res Treat 125:351–362. https://doi.org/10.1007/s10549-010-0822-2
Chang M et al (2005) beta-Adrenoreceptors reactivate Kaposi’s sarcoma-associated herpesvirus lytic replication via PKA-dependent control of viral RTA. J Virol 79:13538–13547. https://doi.org/10.1128/JVI.79.21.13538-13547.2005
Liu J et al (2015) The effect of chronic stress on anti-angiogenesis of sunitinib in colorectal cancer models. Psychoneuroendocrinology 52:130–142. https://doi.org/10.1016/j.psyneuen.2014.11.008
Deng GH et al (2014) Exogenous norepinephrine attenuates the efficacy of sunitinib in a mouse cancer model. J Exp Clin Cancer Res 33:21. https://doi.org/10.1186/1756-9966-33-21
Wei WJ, Shen CT, Song HJ, Qiu ZL, Luo QY (2016) Propranolol sensitizes thyroid cancer cells to cytotoxic effect of vemurafenib. Oncol Rep 36:1576–1584. https://doi.org/10.3892/or.2016.4918
Pon CK, Lane JR, Sloan EK, Halls ML (2016) The beta2-adrenoceptor activates a positive cAMP-calcium feedforward loop to drive breast cancer cell invasion. FASEB J 30:1144–1154. https://doi.org/10.1096/fj.15-277798
Yoshioka Y, Kadoi H, Yamamuro A, Ishimaru Y, Maeda S (2016) Noradrenaline increases intracellular glutathione in human astrocytoma U-251 MG cells by inducing glutamate-cysteine ligase protein via beta3-adrenoceptor stimulation. Eur J Pharmacol 772:51–61. https://doi.org/10.1016/j.ejphar.2015.12.041
Huang XY, Wang HC, Yuan Z, Huang J, Zheng Q (2012) Norepinephrine stimulates pancreatic cancer cell proliferation, migration and invasion via beta-adrenergic receptor-dependent activation of P38/MAPK pathway. Hepato-Gastroenterology 59:889–893. https://doi.org/10.5754/hge11476
Lin X, Luo K, Lv Z, Huang J (2012) Beta-adrenoceptor action on pancreatic cancer cell proliferation and tumor growth in mice. Hepato-Gastroenterology 59:584–588. https://doi.org/10.5754/hge11271
Shan T et al (2011) beta2-adrenoceptor blocker synergizes with gemcitabine to inhibit the proliferation of pancreatic cancer cells via apoptosis induction. Eur J Pharmacol 665:1–7. https://doi.org/10.1016/j.ejphar.2011.04.055
Zhang D, Ma Q, Shen S, Hu H (2009) Inhibition of pancreatic cancer cell proliferation by propranolol occurs through apoptosis induction: the study of beta-adrenoceptor antagonist’s anticancer effect in pancreatic cancer cell. Pancreas 38:94–100. https://doi.org/10.1097/MPA.0b013e318184f50c
Zhang D et al (2011) beta2-adrenoceptor blockage induces G1/S phase arrest and apoptosis in pancreatic cancer cells via Ras/Akt/NFkappaB pathway. Mol Cancer 10:146. https://doi.org/10.1186/1476-4598-10-146
Zhou C et al (2016) Propranolol induced G0/G1/S phase arrest and apoptosis in melanoma cells via AKT/MAPK pathway. Oncotarget 7:68314–68327. https://doi.org/10.18632/oncotarget.11599
Echtay KS et al (2002) Superoxide activates mitochondrial uncoupling proteins. Nature 415:96–99. https://doi.org/10.1038/415096a
Mailloux RJ, Harper ME (2011) Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic Biol Med 51:1106–1115. https://doi.org/10.1016/j.freeradbiomed.2011.06.022
Dal Monte M et al (2014) beta3-adrenergic receptor activity modulates melanoma cell proliferation and survival through nitric oxide signaling. Naunyn Schmiedeberg’s Arch Pharmacol 387:533–543. https://doi.org/10.1007/s00210-014-0969-1
Bruno G et al (2020) beta3-adrenoreceptor blockade reduces tumor growth and increases neuronal differentiation in neuroblastoma via SK2/S1P2 modulation. Oncogene 39:368–384. https://doi.org/10.1038/s41388-019-0993-1
Veiga-Fernandes H, Mucida D (2016) Neuro-Immune Interactions at Barrier Surfaces. Cell 165:801–811. https://doi.org/10.1016/j.cell.2016.04.041
Veiga-Fernandes H, Pachnis V (2017) Neuroimmune regulation during intestinal development and homeostasis. Nat Immunol 18:116–122. https://doi.org/10.1038/ni.3634
Pirzgalska RM et al (2017) Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat Med 23:1309–1318. https://doi.org/10.1038/nm.4422
Qiao G, Chen M, Bucsek MJ, Repasky EA, Hylander BL (2018) Adrenergic Signaling: A Targetable Checkpoint Limiting Development of the Antitumor Immune Response. Front Immunol 9:164. https://doi.org/10.3389/fimmu.2018.00164
Armaiz-Pena GN et al (2015) Adrenergic regulation of monocyte chemotactic protein 1 leads to enhanced macrophage recruitment and ovarian carcinoma growth. Oncotarget 6:4266–4273. https://doi.org/10.18632/oncotarget.2887
Zahalka AH et al (2017) Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science 358:321–326. https://doi.org/10.1126/science.aah5072
Bucsek MJ et al (2017) beta-Adrenergic Signaling in Mice Housed at Standard Temperatures Suppresses an Effector Phenotype in CD8(+) T Cells and Undermines Checkpoint Inhibitor Therapy. Cancer Res 77:5639–5651. https://doi.org/10.1158/0008-5472.CAN-17-0546
Jean Wrobel L et al (2016) Propranolol induces a favourable shift of anti-tumor immunity in a murine spontaneous model of melanoma. Oncotarget 7:77825–77837. https://doi.org/10.18632/oncotarget.12833
Calvani M et al (2019) beta3 -Adrenoceptor as a potential immuno-suppressor agent in melanoma. Br J Pharmacol 176:2509–2524. https://doi.org/10.1111/bph.14660
Chen H et al (2018) Chronic psychological stress promotes lung metastatic colonization of circulating breast cancer cells by decorating a pre-metastatic niche through activating beta-adrenergic signaling. J Pathol 244:49–60. https://doi.org/10.1002/path.4988
Hollmen M, Zheng W, Pollard JW (2019) Editorial: Targeting Myeloid Cells to Fight Cancer. Front Immunol 10:2835. https://doi.org/10.3389/fimmu.2019.02835
Cassetta L et al (2019) Human tumor-associated macrophage and monocyte transcriptional landscapes reveal cancer-specific reprogramming, biomarkers, and therapeutic targets. Cancer Cell 35:588–602 e510. https://doi.org/10.1016/j.ccell.2019.02.009
Sorriento D, Trimarco B, Iaccarino G (2011) Adrenergic mechanism in the control of endothelial function. Transl Med UniSa 1:213–228
Filippi L et al (2015) Infantile hemangiomas, retinopathy of prematurity and cancer: a common pathogenetic role of the beta-adrenergic system. Med Res Rev 35:619–652. https://doi.org/10.1002/med.21336
Ciccarelli M et al (2011) Impaired neoangiogenesis in beta(2)-adrenoceptor gene-deficient mice: restoration by intravascular human beta(2)-adrenoceptor gene transfer and role of NFkappaB and CREB transcription factors. Br J Pharmacol 162:712–721. https://doi.org/10.1111/j.1476-5381.2010.01078.x
Hulsurkar M et al (2017) Beta-adrenergic signaling promotes tumor angiogenesis and prostate cancer progression through HDAC2-mediated suppression of thrombospondin-1. Oncogene 36:1525–1536. https://doi.org/10.1038/onc.2016.319
Iaccarino G et al (2005) Ischemic neoangiogenesis enhanced by beta2-adrenergic receptor overexpression: a novel role for the endothelial adrenergic system. Circ Res 97:1182–1189. https://doi.org/10.1161/01.RES.0000191541.06788.bb
Eelen G et al (2018) Endothelial Cell Metabolism. Physiol Rev 98:3–58. https://doi.org/10.1152/physrev.00001.2017
Morbidelli L et al (1996) Nitric oxide mediates mitogenic effect of VEGF on coronary venular endothelium. Am J Phys 270:H411–H415. https://doi.org/10.1152/ajpheart.1996.270.1.H411
Trochu JN et al (1999) Beta 3-adrenoceptor stimulation induces vasorelaxation mediated essentially by endothelium-derived nitric oxide in rat thoracic aorta. Br J Pharmacol 128:69–76. https://doi.org/10.1038/sj.bjp.0702797
Ferro A et al (1999) Activation of nitric oxide synthase by beta 2-adrenoceptors in human umbilical vein endothelium in vitro. Br J Pharmacol 126:1872–1880. https://doi.org/10.1038/sj.bjp.0702512
Chakroborty D, Sarkar C, Basu B, Dasgupta PS, Basu S (2009) Catecholamines regulate tumor angiogenesis. Cancer Res 69:3727–3730. https://doi.org/10.1158/0008-5472.CAN-08-4289
Yang EV et al (2006) Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res 66:10357–10364. https://doi.org/10.1158/0008-5472.CAN-06-2496
Yang EV et al (2009) Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: implications for stress-related enhancement of tumor progression. Brain Behav Immun 23:267–275. https://doi.org/10.1016/j.bbi.2008.10.005
Park SY et al (2011) Norepinephrine induces VEGF expression and angiogenesis by a hypoxia-inducible factor-1alpha protein-dependent mechanism. Int J Cancer 128:2306–2316. https://doi.org/10.1002/ijc.25589
Wu W et al (2000) Social isolation stress augments angiogenesis induced by colon 26-L5 carcinoma cells in mice. Clin Exp Metastasis 18:1–10. https://doi.org/10.1023/a:1026548715669
Chen H et al (2014) Adrenergic signaling promotes angiogenesis through endothelial cell-tumor cell crosstalk. Endocr Relat Cancer 21:783–795. https://doi.org/10.1530/ERC-14-0236
Dal Monte M et al (2013) Functional involvement of beta3-adrenergic receptors in melanoma growth and vascularization. J Mol Med (Berl) 91:1407–1419. https://doi.org/10.1007/s00109-013-1073-6
Pasquier E et al (2011) Propranolol potentiates the anti-angiogenic effects and anti-tumor efficacy of chemotherapy agents: implication in breast cancer treatment. Oncotarget 2:797–809. https://doi.org/10.18632/oncotarget.343
Bachmann SB et al (2019) A distinct role of the autonomic nervous system in modulating the function of lymphatic vessels under physiological and tumor-draining conditions. Cell Rep 27:3305–3314 e3313. https://doi.org/10.1016/j.celrep.2019.05.050
Panuncio AL, De La Pena S, Gualco G, Reissenweber N (1999) Adrenergic innervation in reactive human lymph nodes. J Anat 194(Pt 1):143–146. https://doi.org/10.1046/j.1469-7580.1999.19410143.x
Raju B, Haug SR, Ibrahim SO, Heyeraas KJ (2007) Sympathectomy decreases size and invasiveness of tongue cancer in rats. Neuroscience 149:715–725. https://doi.org/10.1016/j.neuroscience.2007.07.048
Le CP et al (2016) Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat Commun 7:10634. https://doi.org/10.1038/ncomms10634
Nwabo Kamdje AH et al (2017) Mesenchymal stromal cells’ role in tumor microenvironment: involvement of signaling pathways. Cancer Biol Med 14:129–141. https://doi.org/10.20892/j.issn.2095-3941.2016.0033
Nagaraja AS et al (2017) Adrenergic-mediated increases in INHBA drive CAF phenotype and collagens. JCI Insight 2. https://doi.org/10.1172/jci.insight.93076
Henke E, Nandigama R, Ergun S (2019) Extracellular matrix in the tumor microenvironment and its impact on cancer therapy. Front Mol Biosci 6:160. https://doi.org/10.3389/fmolb.2019.00160
Sood AK et al (2006) Stress hormone-mediated invasion of ovarian cancer cells. Clinical Cancer Res 12:369–375. https://doi.org/10.1158/1078-0432.CCR-05-1698
Gyamfi J, Eom M, Koo JS, Choi J (2018) Multifaceted Roles of Interleukin-6 in Adipocyte-Breast Cancer Cell Interaction. Transl Oncol 11:275–285. https://doi.org/10.1016/j.tranon.2017.12.009
Russell ST, Hirai K, Tisdale MJ (2002) Role of beta3-adrenergic receptors in the action of a tumour lipid mobilizing factor. Br J Cancer 86:424–428. https://doi.org/10.1038/sj.bjc.6600086
Saxton SN, Withers SB, Heagerty AM (2019) Emerging roles of sympathetic nerves and inflammation in perivascular adipose tissue. Cardiovasc Drugs Ther 33:245–259. https://doi.org/10.1007/s10557-019-06862-4
Petruzzelli M et al (2014) A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab 20:433–447. https://doi.org/10.1016/j.cmet.2014.06.011
Cao L et al (2010) Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell 142:52–64. https://doi.org/10.1016/j.cell.2010.05.029
Lu YJ et al (2015) Isoprenaline induces epithelial-mesenchymal transition in gastric cancer cells. Mol Cell Biochem 408:1–13. https://doi.org/10.1007/s11010-015-2477-0
Shan T et al (2014) Novel regulatory program for norepinephrine-induced epithelial-mesenchymal transition in gastric adenocarcinoma cell lines. Cancer Sci 105:847–856. https://doi.org/10.1111/cas.12438
Pu J et al (2017) Adrenaline promotes epithelial-to-mesenchymal transition via HuR-TGFbeta regulatory axis in pancreatic cancer cells and the implication in cancer prognosis. Biochem Biophys Res Commun 493:1273–1279. https://doi.org/10.1016/j.bbrc.2017.09.146
Zhang J et al (2016) Norepinephrine induced epithelial-mesenchymal transition in HT-29 and A549 cells in vitro. J Cancer Res Clin Oncol 142:423–435. https://doi.org/10.1007/s00432-015-2044-9
Lutgendorf SK et al (2018) Biobehavioral modulation of the exosome transcriptome in ovarian carcinoma. Cancer 124:580–586. https://doi.org/10.1002/cncr.31078
Nagaraja AS, Sadaoui NC, Lutgendorf SK, Ramondetta LM, Sood AK (2013) beta-blockers: a new role in cancer chemotherapy? Expert Opin Investig Drugs 22:1359–1363. https://doi.org/10.1517/13543784.2013.825250
Weberpals J, Jansen L, Carr PR, Hoffmeister M, Brenner H (2016) Beta blockers and cancer prognosis – the role of immortal time bias: a systematic review and meta-analysis. Cancer Treat Rev 47:1–11. https://doi.org/10.1016/j.ctrv.2016.04.004
Grytli HH, Fagerland MW, Fossa SD, Tasken KA (2013) Association between use of beta-blockers and prostate cancer-specific survival: a cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur Urol. https://doi.org/10.1016/j.eururo.2013.01.007
Grytli HH, Fagerland MW, Fossa SD, Tasken KA, Haheim LL (2013) Use of beta-blockers is associated with prostate cancer-specific survival in prostate cancer patients on androgen deprivation therapy. Prostate 73:250–260. https://doi.org/10.1002/pros.22564
Lemeshow S et al (2011) beta-blockers and survival among Danish patients with malignant melanoma: a population-based cohort study. Cancer Epidemiol Biomark Prev 20:2273–2279. https://doi.org/10.1158/1055-9965.EPI-11-0249
Udumyan R et al (2017) Beta-blocker drug use and survival among patients with pancreatic adenocarcinoma. Cancer Res 77:3700–3707. https://doi.org/10.1158/0008-5472.CAN-17-0108
De Giorgi V et al (2011) Treatment with beta-blockers and reduced disease progression in patients with thick melanoma. Arch Intern Med 171:779–781. https://doi.org/10.1001/archinternmed.2011.131
Jansen L, Hoffmeister M, Arndt V, Chang-Claude J, Brenner H (2014) Stage-specific associations between beta blocker use and prognosis after colorectal cancer. Cancer 120:1178–1186. https://doi.org/10.1002/cncr.28546
Diaz ES, Karlan BY, Li AJ (2012) Impact of beta blockers on epithelial ovarian cancer survival. Gynecol Oncol 127:375–378. https://doi.org/10.1016/j.ygyno.2012.07.102
Wang HM et al (2013) Improved survival outcomes with the incidental use of beta-blockers among patients with non-small-cell lung cancer treated with definitive radiation therapy. Ann Oncol 24:1312–1319. https://doi.org/10.1093/annonc/mds616
Kokolus KM et al (2018) Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. Oncoimmunology 7:e1405205. https://doi.org/10.1080/2162402X.2017.1405205
Melhem-Bertrandt A et al (2011) Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol Off J Am Soc Clin Oncol 29:2645–2652. https://doi.org/10.1200/JCO.2010.33.4441
Powe DG, Entschladen F (2011) Targeted therapies: Using beta-blockers to inhibit breast cancer progression. Nat Rev Clin Oncol 8:511–512. https://doi.org/10.1038/nrclinonc.2011.123
Botteri E et al (2013) Therapeutic effect of beta-blockers in triple-negative breast cancer postmenopausal women. Breast Cancer Res Treat 140:567–575. https://doi.org/10.1007/s10549-013-2654-3
Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K (2011) Beta blockers and breast cancer mortality: a population- based study. J Clin Oncol Off J Am Soc Clin Oncol 29:2635–2644. https://doi.org/10.1200/JCO.2010.33.5422
Zahalka AH et al (2020) Use of beta-blocker types and risk of incident prostate cancer in a multiethnic population. Urol Oncol. https://doi.org/10.1016/j.urolonc.2020.03.024
Frishman WH (2008) Fifty years of beta-adrenergic blockade: a golden era in clinical medicine and molecular pharmacology. Am J Med 121:933–934. https://doi.org/10.1016/j.amjmed.2008.06.025
Pantziarka P et al (2016) Repurposing Drugs in Oncology (ReDO)-Propranolol as an anti-cancer agent. Ecancermedicalscience 10:680. https://doi.org/10.3332/ecancer.2016.680
Lin CS, Lin WS, Lin CL, Kao CH (2015) Carvedilol use is associated with reduced cancer risk: A nationwide population-based cohort study. Int J Cardiol 184:9–13. https://doi.org/10.1016/j.ijcard.2015.02.015
Horowitz M, Neeman E, Sharon E, Ben-Eliyahu S (2015) Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat Rev Clin Oncol 12:213–226. https://doi.org/10.1038/nrclinonc.2014.224
Neeman E, Zmora O, Ben-Eliyahu S (2012) A new approach to reducing postsurgical cancer recurrence: perioperative targeting of catecholamines and prostaglandins. Clinical Cancer Res 18:4895–4902. https://doi.org/10.1158/1078-0432.CCR-12-1087
Selye H (1975) Stress and distress. Compr Ther 1:9–13
Engler H, Bailey MT, Engler A, Sheridan JF (2004) Effects of repeated social stress on leukocyte distribution in bone marrow, peripheral blood and spleen. J Neuroimmunol 148:106–115. https://doi.org/10.1016/j.jneuroim.2003.11.011
Engler H et al (2004) Effects of social stress on blood leukocyte distribution: the role of alpha- and beta-adrenergic mechanisms. J Neuroimmunol 156:153–162. https://doi.org/10.1016/j.jneuroim.2004.08.005
Palermo-Neto J, de Oliveira Massoco C, Robespierre de Souza W (2003) Effects of physical and psychological stressors on behavior, macrophage activity, and Ehrlich tumor growth. Brain Behav Immun 17:43–54. https://doi.org/10.1016/s0889-1591(02)00057-0
Saul AN et al (2005) Chronic stress and susceptibility to skin cancer. J Natl Cancer Inst 97:1760–1767. https://doi.org/10.1093/jnci/dji401
Sephton S, Spiegel D (2003) Circadian disruption in cancer: a neuroendocrine-immune pathway from stress to disease? Brain Behav Immun 17:321–328. https://doi.org/10.1016/s0889-1591(03)00078-3
Schagen SB et al (2014) Monitoring and optimising cognitive function in cancer patients: Present knowledge and future directions. EJC Suppl 12:29–40. https://doi.org/10.1016/j.ejcsup.2014.03.003
Walker AK et al (2018) Low dose aspirin blocks breast cancer-induced cognitive impairment in mice. PLoS One 13:e0208593. https://doi.org/10.1371/journal.pone.0208593
Engblom C et al (2017) Osteoblasts remotely supply lung tumors with cancer-promoting SiglecF(high) neutrophils. Science 358. https://doi.org/10.1126/science.aal5081
Garofalo C et al (2006) Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clinical Cancer Res 12:1447–1453. https://doi.org/10.1158/1078-0432.CCR-05-1913
Soleyman-Jahi S et al (2019) Attribution of Ghrelin to cancer; attempts to unravel an apparent controversy. Front Oncol 9:1014. https://doi.org/10.3389/fonc.2019.01014
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Magnon, C. (2021). The Adrenergic Nerve Network in Cancer. In: Birbrair, A. (eds) Tumor Microenvironment. Advances in Experimental Medicine and Biology, vol 1329. Springer, Cham. https://doi.org/10.1007/978-3-030-73119-9_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-73119-9_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-73118-2
Online ISBN: 978-3-030-73119-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)