Abstract
Introduction: Dural arteriovenous fistulas (dAVFs) account for 10–15% of all intracranial arteriovenous lesions. Different classification strategies have been proposed in the course of the years. None of them seems to guide the treatment strategy. Objective: We expose the experience of the vascular group at Niguarda Hospital and we propose a very practical classification method based on the location of the shunt. We divide dAVF in sinus and non-sinus in order to simplify our daily practice, as this classification method is simply based on the involvement of the sinuses. Material and Methods: 477 intracranial dural arteriovenous fistulas have been treated. 376 underwent endovascular treatment and 101 underwent surgical treatment. Cavernous sinus DAVFs and Galen ampulla malformations have been excluded from this series as they represent a different pathology per se. 376 dAVFs treated by endovascular approach: 180 were sinus and 179 were non-sinus. 101 dAVFs treated with surgical approach: 15 were sinus and 86 were non-sinus. Discussion: Of the 477 intracranial dAVF the recorded mortality and severe disability was 3% and morbidity less than 4%. All patients underwent a postoperative DSA with nearly 100% of complete occlusion of the fistula. At a mean follow-up of 5 years in one case there was a non-sinus fistula recurrence, due to the presence of a partial clipping of “piè” of the vein. Conclusions: The sinus and non-sinus concept has guided our institution for years and has led to good clinical results. This paper intends to share this practical classification with the neurosurgical community.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Introduction
Dural arteriovenous fistulas (dAVFs) represent 15% of all intracranial arteriovenous shunts. In particular, 7% of supratentorial and 35% of infratentorial shunts are dAVFs [1]. Dural arteriovenous fistulas are a group of acquired pathological vascular malformations, defined by an abnormal communication within the dural leaflets, between arteries and dural venous sinuses and/or subarachnoid veins. The feeding arteries are commonly branches of the external carotid artery, tentorial branches of the internal carotid artery, meningeal branches of the vertebral artery or, rarely, pial branches of the cerebral arteries [2]. Dural arteriovenous fistulas are more commonly supratentorial than infratentorial in location. The transverse-sigmoid sinus junction is the most common location for dAVFs, with a slight left-sided predominance. Through the years many classification systems have been proposed for intracranial dAVFs. These systems are based on the lesion’s venous drainage patterns, as this factor dictates the behavior of the lesion itself. Djindjian and Merland first classified dAVFs according to their venous angioarchitecture in 1977 [3]. In 1995, Cognard further classified both cranial and spinal arteriovenous fistulas according to their venous outflow with prognostic and treatment implications [4]. Borden simplified the Cognard classification, emphasizing that the major factor in predicting an aggressive clinical course is the presence of cortical venous drainage. Unlike venous sinuses, cortical veins are not protected by the dura and cannot withstand arterial pressures. Therefore, dAVFs with cortical venous drainage (Borden types II and III) have a higher risk of rupture and hemorrhage [5, 6].
The Vascular Malformations Study Group of Toronto identified in 1997 the same rate of bleeding for dAVF and cerebral AVMs. Intracranial hemorrhage and neurologic deficit is likely in 2% of the Borden classification type I, 39% of type II, and 79% of type III dAVFs [7]. However, there is a 45% mortality rate over 4 years among patients with cortical venous drainage, a 19.2% intracranial hemorrhage rate per year, and 10.9% new neurologic deficit rate per year. For patients who present with hemorrhage, the rate of repeated hemorrhage is 35% within the first 2 weeks of the initial ictus [1, 6, 8,9,10]. According to our experience [11,12,13], we suggest a simplified classification of dAVFs: sinus and non-sinus. This classification guides the choice of surgical treatment. As previously observed by other authors, posterior fossa localization of the fistulas is not a real adjunctive risk-factor [14].
Our Experience
Sinus Fistula: We define as sinus dAVF all the fistulas having the dural arteries shunting directly into dural sinus. This direct communication between the artery and the sinus can subsequently recruit cerebral veins. The classification for this type of fistula, despite venous recruitment, does not change and still represents a sinus fistula (Fig. 1a).
Non-Sinus Fistula: In non-sinus fistula the sinus maintains functional for the venous discharge of the brain. The pathological shunt is embedded into the dural leaflet without communication with the sinus. Drainage of the fistula depends entirely upon cerebral veins (Fig. 1b).
From 2006 to 2016, at our Neurosurgical Department and our Interventional Neuroradiology at Niguarda Hospital, Milan, Italy, 477 intracranial dural arteriovenous fistulas have been treated: 376 underwent endovascular treatment and 101 underwent surgical treatment. Cavernous sinus dAVFs and Galen ampulla malformations have been excluded from this series, as they represent a different pathology per se. Of the 376 dAVFs treated by the endovascular approach, 180 were sinus and 179 were non-sinus. Of the 101 dAVFs treated with the surgical approach: 15 were sinus and 86 were non-sinus.
This classification was found to be very useful in our daily practice. In a sinus fistula there is a direct communication between the arterious dural branch and one dural sinus, and cerebral veins can subsequently be recruited. These recruited veins, called by some authors “red veins” due to the inverted internal blood flow, are not functioning veins. Red veins must be excluded from the circulation together with the excision of the portion of sinus interested by the fistula. In non-sinus fistula, the shunt is embedded into the dural leaflet and it doesn’t communicate with any sinus, whereas it drains itself into a cortical vein. Therefore, the sinus is functional for the brain. This draining vein—a red vein—has to be occluded proximally (“piè” of the vein) through a surgical or endovascular transvenous approach.
Correct individuation of the type of fistula, sinus or not sinus, is important in order to plan a proper surgical treatment, regardless of the presence of venous varices in the malformation. Those varices, in fact, do not represent an anatomopathological variant and do not require a different surgical approach [13].
The draining vein in a non-sinus fistula, with one or more associated varices on its course, so-called Type IV of some classification systems [4], does not configure a different type of fistula in terms of another anatomopathological entity, so it is not an adjunctive surgical risk factor. Therefore, surgical treatment of both infratentorial and supratentorial fistulas is the same: clipping or occlusion of the vein at its emergence (See “Illustrative Cases”: Fig. 2, Cases 1 and 2)
Illustrative Cases
Case 1
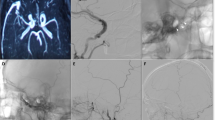
A 65-year-old man come to observation for ictal onset of headache and coma. At admittance in ER, he presented with GCS 3 and anisocoria ( > right), onset <1 h. A CT scan showed a left temporal ICH and a CT-Angio with 3D reconstruction showed a non-sinus dural fistula of pterional dura (a–c). The patient underwent an urgent surgery, with carotid exposure at neck. A wide craniotomy was performed, the hemorrhage was drained, and non-sinus fistula was excluded through multiple clipping of drainage vein, after temporary (7 min) occlusion of external carotid artery (d). The postoperative stay was good, a postoperative DSA showed the complete exclusion of dAVF, and a postoperative CT scan showed the results of previous hemorrhage (e–f). At 2 months follow-up the patient was able to walk alone and he had just a slight expressive dysphasia.
Case 2
A 45-year-old female came to our ED for progressive onset of nucal headache and instability. A first CT scan showed a right, small intracerebellar hemorrhage (g). Six hours after admittance, the patient complained of a new headache attack and the onset of a coma. A new CT scan showed a new hemorrhage and a DSA showed a falco-tentorial non-sinus fistula with associated varix on the drainage vein (h, i, l). The patient underwent urgent surgery with drainage of hemorrhage and clipping of “piè” of the vein. Varix was excised with the hemorrhage (m, n). Postoperative stay was good with a prompt recovering of consciousness. The patient was discharged after 2 weeks with a slight instability.
The different surgical approach in FAVD next to a venous sinus depends on the type of the shunt and it doesn’t depend on the localization but on type of drainage and on the recruited cerebral veins. In the case of a sinus fistula, therefore, it is possible the resection of the fistulous sinus tract and the occlusion of the recruited nonfunctional veins and the absolute preservation of the veins still functional to the brain (See “Illustrative Cases”: Fig. 3, Cases 3 and 4).
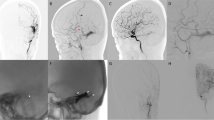
Case 3
A 48-year-old male came to our observation for an intracranial hypertension syndrome with slow onset (some months before), with headache and progressive cognitive impairment. Some days before the patient complained of a severe impairment of awareness and vomiting with associated visual impairment. A DSA showed a sinus fistula of the torcular with engorgement of straight sinus and severe recruitment of many cerebral veins, which seemed to persist long because of engorgement (a–c). The patient underwent a surgical resection of torcular with occlusion of recruited veins (red veins), both not functional for the brain. Postoperative DSE showed exclusion of the FAVD and the non-visualization of straight sinus in arterial phase, for the restoring of a normal situation (d, e).
Case 4
A 17-year-old male with a slow onset symptomatology (6 months) with headache and visual blurring. A DSA showed a sinus FAVD of the middle third of SSS with recruitment of cerebral veins (f–h). The anterior one is not functional; the posterior one, instead, remains normally visible in venous phases of angiography when dAVF is not visible. The patient underwent surgical resection of fistulous sinus tract with occlusion of anterior recruited vein (i, l, m) and avoidance of the posterior one, functional for the brain. The postoperative DSA shows a normal venous angiogram (n).
Different types of dAVFs (sinus or non-sinus) in posterior cranial fossa may localize in the same place, but they require different surgical treatment, in particular those located at the petrous bone apex (See “Illustrative Cases”: Fig. 4, Cases 5 and 6). Finally, non-sinus fistulas with associated varix and perimedullary drainage, so-called Type V of Cognard-Gobin Classification [4], are not a different type of fistula and they do not add an adjunctive surgical risk factor [15] (See “Illustrative Cases”: Fig. 5, Cases 7 and 8).
Case 5
A 44-year-old female fashion designer come to our observation for a progressive brainstem symptomatology. An MRI showed a right ischemic lesion in the pons. Three years before, she was treated in another institution with embolization of a sinus transverse-sigmoid dAVF with an initial recruitment of the superior petrous sinus (a–c). A new DSA showed sinus recanalization associated with an evident recruitment of the superior petrous sinus and reflux in engorged infratentorial veins (d, e). The postoperative angiography showed a disappearance of sinus fistula after sinus removal and clipping of main recruited vein (f). After 6 months of swallowing rehabilitation the patient was able to go back to her job.
Case 6
A 48-year-old male journalist come to our observation for a progressive tetraparesis with sphincter disorders. An MRI showed a severe cervical spine myelopathy, and because of the presence of a disk herniation at C4–C5, initial diagnosis was spondylotic cervical myelopathy. Because of the presence, in MRI, of vascular structures in cervical subarachnoid space (g), a second opinion suggested a complete angiographic study of the brain and medulla. The latter showed a non-sinus fistula of the apex of the right petrous bone, with feeder from the Bernasconi-Cassinari artery and drainage in a perimesencephalic vein with associated engorgement of perimedullary veins (h, i, l). The patient underwent surgical intervention through a suboccipital craniotomy and clipping of drainage vein at the apex of the petrous bone (m, n). Postoperative DSA showed the complete exclusion of the dAVF (o). After 6 months of rehabilitation, the patient went back to work with autonomous life and with slight sphincter impairment.
Case 7
A 52-year-old male presented with a progressive myelopathy, and MRI showed a cervical spine myelopathy without a cervical spondylosis and presence of vascular structures in posterior subarachnoid space (a). The patient underwent complete DSA of brain and medulla, which showed a non-sinus fistula on the dura close to the entrance into intracranial space of the left vertebral artery and engorgement of perimedullary veins of the cervical tract (b). Through a far lateral approach, the “piè” of the vein was exposed in the anterior part of the intracranial entry point of vertebral artery (c, d). The malformation was excluded through clipping of the drainage vein at its origin. Postoperative DSA showed the complete exclusion of the dAVF (e). At 5 months follow-up, the patient walked with aid and still had sphincter disorders.
Case 8
A 58-year-old Italian politician come to our observation for a left tinnitus with onset 1 year previous. Some weeks before, the patient experienced onset of progressive motor impairment in walking with sensitive deficit. Angiography showed a non-sinus fistula close to the jugular foramen (f, g). The fistula was excluded through a surgical intervention with clipping of “piè” of the vein (h, i, l). Post-operative DSA showed the complete disappearance of the malformation (m).
Surgical Results
Surgical series results are described in Table 1. Mortality and severe disability was 3% and morbidity less than 4%. All patients underwent a postoperative DSA with nearly 100% of complete occlusion of the fistula. At a mean follow up of 5 years in one case there was a non-sinus fistula recurrence, due to the presence of a partial clipping of “the shunting foot.” Our surgical experience underlines the fact that posterior fossa localization, per se, does not represent and adjunctive risk factor for treatment. Moreover, based on shunt location, dAVFs can be classified in the same way as meningiomas: falcotentorial, straight sinus-vein of Galen-torcular, transverse and jugular sinus, CPA and foramen magnum (before or at the entrance of the vertebral artery).
Discussion
Dural arteriovenous fistulas (dAVFs) account for 10–15% of all intracranial arteriovenous lesions. Symptoms and prognosis are highly variable. Some dural AVFs produce neurological symptoms depending on the locations and the involved structures; others are associated with intracranial hemorrhage [1, 7, 16]. It is very important to have a good understanding of the natural history of dural AVFs when it comes to decision-making about treatment (See Fig. 6). This challenging and interesting topic has been reviewed since 1984, when Malik et al. [17] studied 223 previously reported cases and concluded that lesions related to large dural sinuses are less likely to bleed than lesions with restricted dural outflow. In this first review there were no angiographic features, in particular, the pattern of venous drainage taken into consideration. In 1986, Lasjaunias et al. [18] presented a meta-analysis of 191 cases. They analyzed the mechanism of neurologic manifestations and concluded that apart from the peripheral cranial nerve palsy due to arterial steal phenomena, central nervous system symptoms seem to be related to passive venous hypertension. In 1990, Awad et al. [19] reviewed 360 cases reported in the literature and 17 of their own cases to compare the angiographic features of 100 aggressive cases and 277 benign cases. They concluded that leptomeningeal venous drainage, variceal or aneurysmal venous dilatations, and galenic drainage were indicative of possible aggressive neurologic signs. In 1995 Cognard et al. [4] reviewed a series of 205 consecutive patients with dural AVFs over 18 years. The purpose was to complete and validate the classification of dural AVFs proposed in 1978 by Djindjian et al. [3]. In the same year Borden proposed his classification system [5], which stratifies lesions on the basis of the site of venous drainage and the presence or absence of cortical venous drainage. Borden type I lesions have the direct communication of meningeal arteries with a meningeal vein or dural venous sinus and exhibit normal antegrade flow. Type II lesions have shunts between the meningeal arteries and dural sinus, with retrograde flow into the subarachnoid veins, causing venous hypertension. Type III lesions have direct drainage of meningeal arteries into subarachnoid veins or an “isolated” sinus segment. The latter phenomenon is often the result of thrombosis on either side of the arterialized sinus segment, which directs retrograde flow into the subarachnoid venous system. The Borden classification scheme further subclassifies lesions as single-hole (a) or multiple-hole (b) fistulas. In our experience, the most useful classification for a merely surgical point of view is to distinguish intracranial dAVFs as sinus or non-sinus, because the simple understanding of this point leads to the correct treatment. On the basis of venous drainage, Borden [5] classified intracranial dAVFs into three main types: sinusal, sinusal with recruitment of one or more cerebral veins, and fistulas draining into cortical cerebral veins.
Localization of posterior cranial fossa dAVFs (See Table 2 for description)
Commonly, type I fistulas have a benign behavior and are conservatively treated. If a disabling bruit is present, a palliative transarterial embolization can be indicated, while surgery is chosen in very selected cases.
However, Type II and III dAVFs usually show an aggressive behavior including hemorrhage and progressive neurological deficits. Davies [1, 7] report an intracranial hemorrhage rate of 19.2% lesion/year and non-hemorrhagic deficit of 10.7% lesion/year. The complete obliteration of Type II or Type III fistula by embolization can be obtained in less than 50% of cases via the transarterial route and in more than 80% of cases when the venous route is used.
Urtasun, Roy, Link and Lucas [20,21,22,23] reported rates of 72% complete obliteration and 28% partial obliteration in patients treated by radiosurgery at 1 and 3 years of follow-up. On the basis of shunt location, posterior cranial fossa dAVFs can be classified in the same way as meningiomas: falcotentorial, straight sinus/vein of Galen, torcular, transverse sinus, jugular sinus, CPA, and foramen magnum dAVFs (Fig. 7). From a practical surgical point of view we suggest to simply classify dAVFs as “sinusal” or “non sinusal fistula.” In the former type there is a direct communication between the dural shunt and one sinus, and cortical veins can sometimes be recruited; in the latter type the shunt is embedded into the dura and the drainage always involves a cerebral vein. As for all diseases, the decision-making process must take into account surgeon experience, aggressiveness of the lesion, and limits of the treatment. Three different approaches can be indicated in dAVFs: surgical, endovascular (i.e., transarterial/transvenous embolization), and radiosurgical with Gamma Knife.
Surgical treatment is different in sinus and non-sinus fistulas and comprise involved sinus resection and clipping or endovascular occlusion of recruited cerebral veins. Recruited veins, the so-called “red veins,” are not functional and show an inverted internal blood flow. Distal to the involved sinus there could be functional blue veins that must be spared. On the other hand, treatment of non-sinusal fistula consists in clipping or endovascular occlusion of the draining red vein which, as mentioned before, is a non-functional vein.
Conclusions
We want to underline the role of venous drainage in the management of dAVFs. Prioritization of treatment options is crucial to achieving a good result. As a consequence, identifying sinus vs non-sinus fistulas is crucial from a surgical point of view. Sinus fistulas require sinus excision and clipping and subsequent occlusion of recruited veins. Non-sinus fistulas require, on the other hand, clipping or endovascular venous embolization of the drainage vein. A posterior cranial fossa location does not represent an adjunctive risk factor in the treatment of dural fistulas. Type IV and V in Cognard and Gobin’s classification are not to be considered as independent entities: varices and perimedullary drainage can be seen in both sinus and non-sinus fistulas.
References
Davies MA, Saleh J, Ter Brugge K, Willinsky R, Wallace MC (1997) The natural history and management of intracranial dural arteriovenous fistulae. Part 1: benign lesions. Interv Neuroradiol 3(4):295–302
Miller NR (2012) Dural carotid-cavernous fistulas: epidemiology, clinical presentation, and management. Neurosurg Clin N Am 23:179–192
Djindjian R, Merland JJ, Theron J (1978) Super-selective arteriography of the external carotid artery. Springer-Verlag, New York
Cognard C, Gobin YP, Pierot L et al (1995) Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology 194(3):671–680
Borden JA, Wu JK, Shucart WA (1995) A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg 82:166–179
Liu JK, Dogan A, Ellegala DB, Carlson J, Nesbit GM, Barnwell SL, Delashaw JB (2009) The role of surgery for high-grade intracranial dural arteriovenous fistulas: importance of obliteration of venous outflow. J Neurosurg 110:913–920
Davies MA, Saleh J, Ter Brugge K, Willinsky R, Wallace MC (1997) The natural history and management of intracranial dural arteriovenous fistulae. Part 2: aggressive lesions. Interv Neuroradiol 3(4):303–311
Gandhi D et al (2012) Intracranial dural arteriovenous fistulas: classification, imaging findings, and treatment. AJNR Am J Neuroradiol 33(6):1007–1013. https://doi.org/10.3174/ajnr.A2798
Huang L et al (2017) Correlation of aggressive intracranial lesions and venous reflux patterns in dural arteriovenous fistulas. World Neurosurg 107:130–136. https://doi.org/10.1016/j.wneu.2017.07.142
Takai K et al (2013) Three-dimensional angioarchitecture of spinal dural arteriovenous fistulas, with special reference to the intradural retrograde venous drainage system. J Neurosurg Spine 18(4):398–408. https://doi.org/10.3171/2013.1.SPINE12305
Collice M, D’Aliberti G et al (2000) Surgical treatment of intracranial dural arteriovenous fistulae: role of venous drainage. Neurosurgery 47(1):56–66. discussion 66–7
Collice M, D’Aliberti G et al (1996) Surgical interruption of leptomeningeal drainage as treatment for intracranial dural arteriovenous fistulas without dural sinus drainage. J Neurosurg 84(5):810–817
D’Aliberti G, Talamonti G, Collice M (1996) Sinus skeletonization. J Neurosurg 85(4):738–740
Lanzino G, Boccardi E (2009) Posterior fossa dural arteriovenous fistulas. J Neurosurg 111(5):887–888
Versari PP, D’Aliberti G, Talamonti G, Branca V, Boccardi E, Collice M (1993) Progressive myelopathy caused by intracranial dural arteriovenous fistula: report of two cases and review of the literature. Neurosurgery 33(5):914–918. discussion 918–9
Newton T, Cronqvist S (1969) Involvement of the dural arteries in intra-cranial arteriovenous malformations. Radiology 90:27–35
Malik G, Pearce J, Ausman J, Mehta B (1984) Dural artenovenous malformations and intracranial hemorrhage. Neurosurgery 15:332–338
Lasjaunias P, Chiu M, Brugge KT, Tolia A, Hurth M, Berenstein M (1986) Neurological manifestations of intracranial dural arterio-venous malformations. J Neurosurg 64:724–730
Awad I, Little J, Akrawi W, Ahl J (1990) Intra-cranial dural arteriovenous malformations: factors predisposing to an aggressive neurological course. J Neurosurg 72:839–850
Link MJ, Coffey RJ, Nichols DA, Gorman DA (1996) The role of radiosurgery and particulate embolization in the treatment of dural arteriovenous fistulas. J Neurosurg 84(5):804–809
Roy D, Raymond J (1997) The role of transvenous embolization in the treatment of intracranial dural arteriovenous fistulas. Neurosurgery 40(6):1133–1141. discussion 1141–4
Urtasun F, Biondi A, Casaco A, Houdart E, Caputo N, Aymard A, Merland JJ (1996) Cerebral dural arteriovenous fistulas: percutaneous transvenous embolization. Radiology 199(1):209–217
Lucas CP, Zabramski JM, Spetzler RF, Jacobowitz R (1997) Treatment for intracranial dural arteriovenous malformations: a meta-analysis from the English language literature. Neurosurgery 40:1119–1130. discussion 1130–1132
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2021 The Author(s)
About this paper
Cite this paper
D’Aliberti, G. et al. (2021). Intracranial Dural Arteriovenous Fistulas: The Sinus and Non-Sinus Concept. In: Esposito, G., Regli, L., Cenzato, M., Kaku, Y., Tanaka, M., Tsukahara, T. (eds) Trends in Cerebrovascular Surgery and Interventions. Acta Neurochirurgica Supplement, vol 132. Springer, Cham. https://doi.org/10.1007/978-3-030-63453-7_17
Download citation
DOI: https://doi.org/10.1007/978-3-030-63453-7_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-63452-0
Online ISBN: 978-3-030-63453-7
eBook Packages: MedicineMedicine (R0)