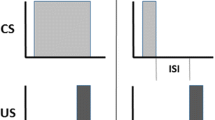
Abstract
In this paper, we simulate the effects of hippocampal lesions on Pavlovian conditioning with an existing neural network model. According to the model, the hippocampus sends a diffuse discrepancy signal that modulates efficacies of synapses from primary sensory to polysensory areas. We hypothesize that this signal lessens the detrimental effects of momentary nonreinforcement and weaker cues on such efficacies in Pavlovian conditioning. Hippocampal lesions are thus hypothesized to exacerbate both detrimental effects. To test this hypothesis against some relevant animal evidence, we ran two computer simulations using simple feedforward neural networks with two hidden layers, designed according to the model. Hippocampal lesions were simulated by removing the networks’ hippocampal units. Networks were trained in various conditions involving momentary nonreinforcement and reinforcement of differently salient cues. The results were reasonably consistent with animal evidence that hippocampal lesion is more disruptive of long-delay than short-trace conditioning (Simulation 1) and backward more than contiguous-trace conditioning (Simulation 2). Implications, limitations, and future directions are discussed.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Calvin NT, McDowell JJ. Unified-theory-of-reinforcement neural networks do not simulate the blocking effect. Behav Process. 2015;120:54–63. https://doi.org/10.1016/j.beproc.2015.08.008.
Donahoe JW, Burgos JE, Palmer DC. A selectionist approach to reinforcement. J Exp Anal Behav. 1993;60(1):17–40. https://doi.org/10.1901/jeab.1993.60-17.
Burgos JE, Donahoe JW. Unified principle of reinforcement: a reply to N. T. Calvin and J. J. McDowell. Behav Process. 2016;126:46–54. https://doi.org/10.1016/j.beproc.2016.03.003.
Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20(1):11–21. https://doi.org/10.1136/jnnp.20.1.11.
Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci. 1986;6(10):2950–67. https://doi.org/10.1523/JNEUROSCI.06-10-02950.1986.
Akase E, Alkon DL, Disterhoft JF. Hippocampal lesions impair memory of short-delay conditioned eye blink in rabbits. Behav Neurosci. 1989;103(5):935–43. https://doi.org/10.1037/0735-7044.103.5.935.
Berger TW, Orr WB. Hippocampectomy selectively disrupts discrimination reversal conditioning of the rabbit nictitating membrane response. Behav Brain Res. 1983;8(1):49–68. https://doi.org/10.1016/0166-4328(83)90171-7.
Beylin AV, Gandhi CC, Wood GE, Talk AC, Matzel LD, Shors TJ. The role of the hippocampus in trace conditioning: temporal discontinuity or task difficulty? Neurobiol Learn Mem. 2001;76(3):447–61. https://doi.org/10.1006/nlme.2001.4039.
Chowdhury N, Quinn JJ, Fanselow MS. Dorsal hippocampus involvement in trace fear conditioning with long, but not short, trace intervals in mice. Behav Neurosci. 2005;119(5):1396–402. https://doi.org/10.1037/0735-7044.119.5.1396.
Clark RE, Squire LR. Classical conditioning and brain systems: the role of awareness. Science. 1998;280(5360):77–81. https://doi.org/10.1126/science.280.5360.77.
Moyer JR, Deyo RA, Disterhoft JF. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav Neurosci. 1990;104(2):243–52. https://doi.org/10.1037/0735-7044.104.2.243.
Port RL, Mikhail AA, Patterson MM. Differential effects of hippocampectomy on classically conditioned rabbit nictitating membrane response related to interstimulus interval. Behav Neurosci. 1985;99(2):200–8. https://doi.org/10.1037/0735-7044.99.2.200.
Schmaltz LW, Theios J. Acquisition and extinction of a classically conditioned response in hippocampectomized rabbits (Oryctolagus cuniculus). J Comp Physiol Psychol. 1972;79(2):328–33. https://doi.org/10.1037/h0032531.
Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Behav Neurosci. 1986;100(5):729–44. https://doi.org/10.1037/0735-7044.100.5.729.
Ludvig EA, Sutton RS, Verbeek E, Kehoe EJ. A computational model of hippocampal function in trace conditioning. Adv Neural Inf Process Syst (NIPS-08). 2009;21:993–1000. Available from: https://papers.nips.cc/paper/3619-a-computational-model-of-hippocampal-function-in-trace-conditioning.
Rodriguez P, Levy WB. A model of hippocampal activity in trace conditioning: where is the trace? Behav Neurosci. 2001;115(6):1224–38. https://doi.org/10.1037/0735-7044.115.6.1224.
Yamazaki T, Tanaka S. A neural network model for trace conditioning. Int J Neural Syst. 2005;15(1–2):23–30. https://doi.org/10.1142/S0129065705000037.
Schmajuk NA, DiCarlo JJ. A neural network approach to hippocampal function in classical conditioning. Behav Neurosci. 1991;105(1):82–110. https://doi.org/10.1037/0735-7044.105.1.82.
Moustafa AA, Wufong E, Servatious RJ, Pang KCH, Gluck MA, Myers CE. Why trace and delay conditioning are sometimes (but not always) hippocampal dependent: a computational model. Brain Res. 2013;1493:48–67. https://doi.org/10.1016/j.brainres.2012.11.020.
Gluck MA, Myers CE. Hippocampal mediation of stimulus representation: a computational theory. Hippocampus. 1993;3(4):491–516. https://doi.org/10.1002/hipo.450030410.
Quinn JJ, Oommen SS, Morrison GE, Fanselow MS. Post-training excitotoxic lesions of the dorsal hippocampus attenuate forward trace, backward trace, and delay fear conditioning in a temporally specific manner. Hippocampus. 2002;12(4):495–504. https://doi.org/10.1002/hipo.10029.
Bangasser DA, Waxler DE, Santollo J, Shors TJ. Trace contiguity and the hippocampus: the importance of contiguity. J Neurosci. 2006;26(34):8702–6. https://doi.org/10.1523/JNEUROSCI.1742-06.2006.
Burgos JE. The operant/respondent distinction: a computational neural-network analysis. In: Schmajuk N, editor. Computational models of conditioning. Cambridge: Cambridge University Press; 2010.
Burns R, Burgos JE, Donahoe JW. Pavlovian conditioning: pigeon nictitating membrane. Behav Process. 2011;86:102–8. https://doi.org/10.1016/j.beproc.2010.10.004.
Burgos JE. Evolving artificial neural networks in Pavlovian environments. In: Donahoe JW, Dorsel-Packard V, editors. Neural-network models of cognition: biobehavioral foundations. Amsterdam: Elsevier; 1997. https://doi.org/10.1016/S0166-4115(97)80090-8.
Burgos JE, Donahoe JW. Structure and function in selectionism: implications for complex behavior. In: Leslie J, Blackman D, editors. Issues in experimental and applied analyses of human behavior. Reno: Context Press; 2000.
Burgos JE, Flores C, García O, Díaz C, Cruz Y. A simultaneous procedure facilitates acquisition under an optimal interstimulus interval in artificial neural networks and rats. Behav Process. 2008;78(2):302–9. https://doi.org/10.1016/j.beproc.2008.02.018.
Donahoe JW, Burgos JE. Behavior analysis and revaluation. J Exp Anal Behav. 2000;74(3):331–46. https://doi.org/10.1901/jeab.2000.74-331.
Burgos JE. Theoretical note: simulating latent inhibition with selection neural networks. Behav Process. 2003;62(1–3):183–92. https://doi.org/10.1016/S0376-6357(03)00025-1.
Burgos JE. Theoretical note: the C/T ratio in artificial neural networks. Behav Process. 2005;69(2):249–56. https://doi.org/10.1016/j.beproc.2005.02.008.
Burgos JE. Autoshaping and automaintenance: a neural-network approach. J Exp Anal Behav. 2007;88(1):115–30. https://doi.org/10.1901/jeab.2007.75-04.
Sánchez JM, Galeazzi JM, Burgos JE. Some structural determinants of Pavlovian conditioning in artificial neural networks. Behav Process. 2010;84(1):526–35. https://doi.org/10.1016/j.beproc.2010.01.018.
Burgos JE, Murillo-Rodríguez E. Neural-network simulations of two context-dependence phenomena. Behav Process. 2007;75(2):242–9. https://doi.org/10.1016/j.beproc.2007.02.003.
Burgos JE, García-Leal Ó. Autoshaped choice in artificial neural networks: implications for behavioral economics and neuroeconomics. Behav Process. 2015;114:63–71. https://doi.org/10.1016/j.beproc.2015.01.010.
Burgos JE. Misbehavior in artificial neural networks. Int J Comp Psychol. 2015;28:1–21. Available from: http://escholarship.org/uc/item/3vb500tv.
Ji J, Maren S. Electrolytic lesions of the dorsal hippocampus disrupt renewal of conditional fear after extinction. Learn Mem. 2005;12(3):270–6. https://doi.org/10.1101/lm.91705.
McEchron MD, Tseng W, Disterhoft JF. Single neurons in CA1 hippocampus encode trace interval duration during trace heart rate (fear) conditioning in rabbit. J Neurosci. 2003;23(4):1535–47. https://doi.org/10.1523/JNEUROSCI.23-04-01535.2003.
Moyer JR, Power JM, Thompson LT, Disterhoft JF. Increased excitability of aged rabbit CA1 neurons after trace eyeblink conditioning. J Neurosci. 2000;20(14):5476–82. https://doi.org/10.1523/JNEUROSCI.20-14-05476.2000.
Frey U, Schroeder H, Matthies H. Dopaminergic antagonists prevent long-term maintenance of posttetanic LTP in the CA1 region of rat hippocampal slices. Brain Res. 1990;522(1):69–75. https://doi.org/10.1016/0006-8993(90)91578-5.
Gasbarri A, Packard MG, Campana E, Pacitti C. Anterograde and retrograde tracing of projections from the ventral tegmental area to the hippocampal formation in the rat. Brain Res Bull. 1994;33(4):445–52. https://doi.org/10.1016/0361-9230(94)90288-7.
Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci. 2003;6(5):526–31. https://doi.org/10.1038/nn1049.
Morris RGM, Moser EI, Riedel G, Martin SJ, Sandin J, Day M, O’Carroll C. Elements of a neurobiological theory of the hippocampus: the role of activity-dependent synaptic plasticity in memory. Philos Trans R Soc Lond Ser B Biol Sci. 2003;358(1432):773–86. https://doi.org/10.1098/rstb.2002.1264.
Scatton B, Simon H, Le Moal M, Bischoff S. Origin of dopaminergic innervation of the rat hippocampal formation. Neurosci Lett. 1980;18(2):125–31. https://doi.org/10.1016/0304-3940(80)90314-6.
Verney C, Baulac M, Berger B, Alverez C, Vigny A, Helle KB. Morphological evidence for a dopaminergic terminal field in the hippocampal formation of young and adult rat. Neuroscience. 1985;14(4):1039–52. https://doi.org/10.1016/0306-4522(85)90275-1.
Stein L, Belluzzi JD. Cellular investigations of behavioral reinforcement. Neurosci Biobehav Rev. 13(2–3):69–80. https://doi.org/10.1016/S0149-7634(89)80014-4.
Amaral DG, Insausti R, Cowan WM. Evidence for a direct projection from the superior temporal gyrus to the entorhinal cortex in the monkey. Brain Res. 1983;275(2):263–77. https://doi.org/10.1016/0006-8993(83)90987-3.
Burwel RD, Amaral DG. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J Comp Neurol. 398(2):179–205. https://doi.org/10.1002/(SICI)1096-9861(19980824)398:2%3C179::AID-CNE3%3E3.0.CO;2-Y.
Mohedano-Moriano A, Martínez-Marcos A, Pro-Sistiaga P, Blaizot X, Arroyo-Jimenez MM, Marcos P, Artacho-Pérula E, Insausti R. Convergence of unimodal and polymodal sensory input to the entorhinal cortex in the fascicularis monkey. Neuroscience. 2008;151(1):255–71. https://doi.org/10.1016/j.neuroscience.2007.09.074.
Van Hoesen GW, Hyman BT. Hippocampal formation: anatomy and the patterns of pathology in Alzheimer’s disease. Prog Brain Res. 1990;83:445–57. https://doi.org/10.1016/S0079-6123(08)61268-6.
Swanson LW, Köhler C. Anatomical evidence for direct projections from the entorhinal area to the entire cortical mantle in the rat. J Neurosci. 6(10):3010–23. http://www.jneurosci.org/content/6/10/3010.long.
Barbas H, Blatt GJ. Topographically specific hippocampal projections target functionally distinct prefrontal areas in the Rhesus monkey. Hippocampus. 1995;5(6):511–33. https://doi.org/10.1002/hipo.450050604.
Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46(5):703–13. https://doi.org/10.1016/j.neuron.2005.05.002.
Witnauer JE, Miller RR. Conditioned suppression is an inverted-U function of footshock intensity. Learn Behav. 2013;41(1):94–106.
Marlin NA. Contextual associations in trace conditioning. Anim Learn Behav. 1981;9(4):519–23. https://doi.org/10.3758/BF03209784.
G-y W, Yao J, Hu B, H-m Z, Li Y-d, Li X, Li Q, J-f S. Reevaluating the role of the hippocampus in delay eyeblink conditioning. PLoS One. 2013;8:e71249. https://doi.org/10.1371/journal.pone.0071249.
Grossberg S. A neural model of attention, reinforcement, and discrimination learning. Int Rev Neurobiol. 1975;18:263–327. https://doi.org/10.1016/S0074-7742(08)60037-9.
Schmajuk NA, DiCarlo JJ. Stimulus configuration, classical conditioning, and hippocampal function. Psychol Rev. 1992;99(2):268–305. https://doi.org/10.1037/0033-295X.99.2.268.
Chang RC, Blaisdell AP, Miller RR. Backward conditioning: mediation by the context. J Exp Psychol Anim Behav Process. 2003;29(3):171–83. https://doi.org/10.1037/0097-7403.29.3.171.
Chang RC, Stout S, Miller RR. Comparing excitatory backward and forward conditioning. Q J Exp Psychol. 2004;57B(1):1–23. https://doi.org/10.1080/02724990344000015.
Heth CD, Rescorla RA. Simultaneous and backward fear conditioning in the rat. J Comp Physiol Psychol. 1973;82(3):434–43.
Spetch ML, Wilkie DM, Pinel JPJ. Backward conditioning: a reevaluation of the empirical evidence. Psychol Bull. 1981;89(1):163–75.
Kaye H, Pearce JM. Hippocampal lesions attenuate latent inhibition of a CS and a neutral stimulus. Psychobiology. 1987;15(1):293–9.
Coutureau E, Galani R, Gosselin O, Majchrzak M, Di Scala G. Entorhinal but not hippocampal or subicular lesions disrupt latent inhibition in rats. Neurobiol Learn Mem. 1999;72(3):143–57. https://doi.org/10.1006/nlme.1998.3895.
Solomon PR. Role of the hippocampus in blocking and conditioned inhibition of the rabbit’s nictitating membrane response. J Comp Physiol Psychol. 1977;91(2):407–17. https://doi.org/10.1037/h0077330.
Garrud P, Rawlins NP, Mackintosh NJ, Goodal G, Cotton MM, Feldon J. Successful overshadowing and blocking in hippocampectomized rats. Behav Brain Res. 1984;12(1):39–53. https://doi.org/10.1016/0166-4328(84)90201-8.
Good M, Macphail EM. Hippocampal lesions in pigeons (Columba livia) disrupt preexposure but not overshadowing or blocking. Quart J Exp Psychol. 1994;47B(3):263–91. https://journals.sagepub.com/doi/abs/10.1080/14640749408401360.
Holland PC, Fox GD. Effects of hippocampal lesions on overshadowing and blocking procedures. Behav Neurosci. 2003;117(3):650–6. https://doi.org/10.1037/0735-7044.117.3.650.
Rickert EJ, Bent TL, Lane P, French J. Hippocampectomy and the attenuation of blocking. Behav Biol. 1978;22(2):147–60. https://doi.org/10.1016/S0091-6773(78)92170-3.
Schmajuk NA, Spear NE, Isaacson RL. Absence of overshadowing in rats with hippocampal lesions. Physiol Psychol. 1983;11(1):59–62. https://doi.org/10.3758/BF03326770.
Treviño M. Associative learning through acquired salience. Front Behav Neurosci. 2016;11(9):353. https://doi.org/10.3389/fnbeh.2015.00353.
Treviño M, Oviedo T, Jendritza P, Li S-B, Köhr G, De Marco RJ. Controlled variations in stimulus similarity during learning determine visual discrimination capacity in freely moving mice. Sci Rep. 2013;3:1048. https://doi.org/10.1038/srep01048.
Gerstner W, Sprekeler H, Deco G. Theory and simulation in neuroscience. Science. 2012;338(6103):60–5. https://doi.org/10.1126/science.1227356.
Ethical Approval
No animals were used or participated in this article, excepting the authors, who were not mistreated in anyway; nor does it cite any studies with animals performed by any of the authors. Whether or not the networks used were mistreated requires an expanded code of ethics that includes the possibility and nature of suffering in artificial systems. Unfortunately, no so such code is yet available, and the issue of whether silicon computer quantum states are capable of suffering remains controversial.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Appendix
Appendix
Activation Rule
The model’s two main equations are the activation rule and the learning rule. The activation rule is used to compute the momentary level of activation of a neural processing unit j at moment t. The learning rule is used to compute the change in the weight of a connection from afferent (pre-connection, presynaptic) unit i to target (post-connection, postsynaptic) unit j. The activation rule is defined as follows:
where j is a neural processing (hidden, output, H, or VD) unit, t is a moment in time, and
is the logistic function with constant mean μ = 0.5 and standard deviation σ = 0.1 (a spontaneous activation free parameter). In this function
for excj,t, and
for inhj,t, where m denotes the total number of excitatory units connected to j and n the total number of inhibitory units connected to j. No inhibitory units were used in this study, so the amount of inhibition was 0.0 for all units and networks in all simulations. Whether the rule is in reactivation or decay mode at t depends on a Gaussian threshold (θj,t), a random number generated according to a Gaussian distribution with a mean of 0.2 and standard deviation of 0.15. θj,t is dynamical, as it is generated at every moment for every computational unit. The other two activation free parameters are temporal summation (τj = 0.1) and decay (κ j = 0.1). The same free parameters that were used here have been used in most previous simulation research with the model.
On input activations and stimulus traces. A network’s inputs in this model are not activated via the activation rule but manually, by just setting their activations according to some training protocol that simulates a conditioning procedure of interest. The protocol includes simulations of sensory stimuli typically used as cues in conditioning studies (e.g., lights, tones, noises, etc.) and biologically significant stimuli typically used as USs or primary reinforcers (e.g., food, water, electrical shocks, etc.). In previous research with this model, we have assumed that these activations are real-time primary sensory effects of external stimuli. Hence, we do not intend input activations in this model to simulate “traces” qua input activations in the absence of external stimuli but real-time effects of ongoing stimuli. Thus, a greater-than-zero input activation means in this model that a stimulus is effectively present, roughly at that moment.
Thus far in research with this model, we have had no need to conceive input activations as “stimulus traces.” The notion of a stimulus trace in models of Pavlovian conditioning was introduced ad hoc to account for trace conditioning in a way that fits the conventional wisdom that the CS always acquires some associative strength, even if absent. In Simulation 1, however, we simulated trace conditioning (and in Simulation 2, backward conditioning) as only context conditioning. We know this to be extreme and against conventional wisdom, but it is consistent with the evidence (see Marlin, 1981).
Learning Rule
The learning rule is defined as follows:
where α (rate of weight increment) and β (the rate of weight decrement) denote the two free parameters of the rule (α = 0.5 and β = 0.1 for all connections; the same parameters have been used in most previous simulation research with this model). Ideally, the relative values of various parameters would reflect independent, experimentally determined values. All initial weights were set to 0.1 (cf. Burgos 2007).
The other terms of the learning rule are:
-
ai,t: activation of afferent unit (i), either excitatory or inhibitory
-
aj,t: activation of target unit (j)
-
dt = dH,t = |aH,t − aH,t−1| + dD,t(1 − dH,t−1), if j is an S″ or H unit (see Fig. 23.1 for the different kinds of units and how they are connected)
-
dt = dD,t = aD,t − aD,t−1, if j is an M″, D, or M′ unit; if dD,t < 0.0, then dD,t = 0.0
$$ {p}_{i,t}=\frac{a_{i,t}{w}_{i,j,t-1}}{N},\kern1em \mathrm{where}\kern0.5em N={exc}_{j,t}\kern0.5em \mathrm{or}\kern0.5em N={inh}_{j,t} $$ -
depending on whether i is excitatory or inhibitory, respectively
The key factor is dt, a signal that modulates changes of all weights in the same moment (i.e., it is a diffuse signal), inspired by evidence on the roles of hippocampal (e.g., CA1, simulated by H units in the H architecture at the top of Fig. 23.1) and dopaminergic (e.g., ventral tegmental area, simulated by the D unit in Fig. 23.1) areas in conditioning. dt also is a discrepancy signal in that it is defined as a temporal difference between the actual activations of H and D units in successive pairs of moments. The learning rule includes two such modulating signals: dH,t, which depends on the activations of the H units, and dD,t, which depends on the activations of the D units. As shown above, dH,t is amplified by dD,t. However, in the ~H networks (bottom panel, Fig. 23.1), dH,t = 0.0, for which dt = dD,t. Hence, dt in the ~H networks tends to be weaker than in the H networks.
The pi,t and rj,t factors introduce a “rich get richer” sort of competition among connections for a limited amount of weight (1.0) on a common target unit. In the network architectures used in the simulations (see Fig. 23.1), this competition took place on units that received two connections (viz., all the S″ and M″ units, as well as the D unit). The pi,t factor, like some other models, includes a Hebbian component where connection weights partly depend on the co-activations of the connected (afferent and target) units.
In general, connections tend to gain weight (to a greater or lesser degree, depending on several factors) when S* (see Fig. 23.1) is activated and lose weight when S* is not activated. Successive timesteps with a zero S* activation thus promote weight loss. The same learning rule was used to modify the connection weights across all times, connections, networks, units, and training protocols.
All activations and weights are updated at every moment t according to an asynchronous random procedure. In this procedure, a randomly ordered list of all units (or connections) is generated at t, and new activations (or weights) are computed in that order (according to Eq. 23.1 for activations or Eq. 23.2 for weights). The activations (or weights) from t − 1 are immediately replaced by the new activations at t. Hence, by chance, the activation of a unit at t could depend on the activations of its afferents (some or all) at t − 1. Therefore, the propagation of activations across the network, from input to hidden to output layers , is not strictly sequential and synchronous.
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Burgos, J.E., Galeazzi, J.M. (2021). Neural Network Simulations of a Possible Role of the Hippocampus in Pavlovian Conditioning. In: Gargiulo, P.Á., Mesones Arroyo, H.L. (eds) Psychiatry and Neuroscience Update. Springer, Cham. https://doi.org/10.1007/978-3-030-61721-9_23
Download citation
DOI: https://doi.org/10.1007/978-3-030-61721-9_23
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-61720-2
Online ISBN: 978-3-030-61721-9
eBook Packages: MedicineMedicine (R0)