Abstract
Scars can be classified into immature scars and mature scars. Mature scars can be “normal,” atrophic, or hypertrophic. Keloids occur in patients with a genetic predisposition and behave differently than hypertrophic scars, although there can be a continuum in terms of appearance. The molecular mechanisms of scarring, hypertrophic scar and keloids, have been the subject of intensive research. There are still many unanswered questions.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
There has been a wide variety of therapies proposed for the treatment of scars, most of them with a lack of firm randomized controlled clinical trials to support their efficacy, and among other deficiencies, there has been often a lack of appropriate labeling or classification of scars to allow optimal evaluation of existing literature. There is a real benefit to a consistent classification of different types of scars so that different clinicians use a consistent vocabulary allowing a systematic evaluation of treatments and outcomes. One problem has always been the changes in scars over time so that improvements may not necessarily be due to the treatment intervention but simply scar maturation.
Scar classifications have previously been published including notably the 2002 publication in PRS which gained widespread acceptance because it represented the consensus of 12 experts from an international group incorporating Europe, North America, and Australia. In subsequent publications, this classification has been found to sound and not needing further modification. We will use this classification again in this updated textbook.
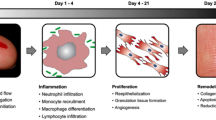
Scarring is the inevitable consequence of tissue injury as opposed to tissue regeneration which is the true restoration of the normal architecture of the skin. True tissue regeneration after injury occurs only in the fetus during the first two trimesters and in amphibians who can even regenerate amputated limbs. In the optimal outcome of a thin flat thin linear scar, the sequence of tissue repair after injury is tightly regulated. After initial platelet aggegation, provisional matrix is deposited, followed by influx of inflammatory cells and subsequent cell proliferation including fibroplasia and angiogenesis. Wound healing overlap, and are followed by cellular apoptosis with resolution of inflammation. Permanent matrix deposition (collagen) begins within 3 days. Maximal collagen deposition occurs in the first few weeks with a combination of type 1 and type 3, followed by many months of collagen breakdown and synthesis with increasing type 1 collagen with increased organization and scar strength. These phases of inflammation, cell proliferation and collagen remodeling result in a fine line scar in the scenario of an incison (“normal” scar), and a wider flat scar in the scenario of an injury over a broader area.
1 Immature Scar
Even a normal scar goes through a period when it is immature , meaning it is pink, often with a healing ridge (edema plus collagen synthesis). Collagen accumulation typically peaks about 3 weeks after surgery and then goes through about 6 months of remodeling with steadily increasing collagen organization, conversion from a mix of type 1 and type 3 collagen to almost entirely type 1 collagen with increased cross-linking, and continued increase in tensile strength. The gains in scar strength are due to improvement in collagen organization and cross-linking rather than an increase in collagen. A useful analogy is to compare the difference between raw wool from a sheared sheep, versus the fine knit of a woolen coat. The amount of wool is the same, but the woven wool is far stronger. However, although the strength of a scar is maximal in approximately 6 months, complete scar maturation as measured by resolution of erythema typically takes a year or even longer. In a human volunteer study conducted by Renovo, superficial scars took longer than a year for the erythema to fully resolve in one-third of patients. In scars in thicker skin with more depth, or in a group of patients with more active scarring process, it probably takes even longer. In my own clinical practice with approximately 20,000 patients of all ages and ethnic backgrounds, my experience mirrors the study by Renovo (◘ Table 9.1).
The resolution of erythema is a useful marker of scar maturity (◘ Fig. 9.1). In all scars, erythema eventually resolves, but in a few percent of patients, I have seen it take longer than 2 years and in a rare hypertrophic scar as long as 10 years. In a study we conducted on burn patients, we found that the average scar elasticity (a measure of collagen remodeling), as measured by a device that applied a constant force to the skin and measured how much it stretched, continued to increase over 5 years (Bartell et al). Although the patients were not followed longitudinally, we simply correlated elasticity to the age of the scar, and it can be inferred that it can take a long time indeed for a scar to reach full maturity.
In an immature scar histologically, there are an increased number of inflammatory cells. After 2 weeks or so, the predominant inflammatory cells are macrophages with scattered lymphocytes and an occasional mast cell. There are increased numbers of fibroblasts including myofibroblasts, increased numbers of blood vessels, and for a period of up to a few months epidermal hyperplasia. The visual appearance is a scar that is erythematous and slightly raised due to increased fluid in the tissues and increased collagen as well as increased cellularity.
2 Mature Scar
In undergoing the transition from an immature scar to a mature scar , the key visual marker is resolution of erythema (◘ Fig. 9.2). At this point, the inflammatory cells, endothelial cells, and most of the fibroblasts have undergone apoptosis, and the epithelium looks completely normal as compared to the adjacent unwounded skin. What is left is a band of collagen fibers that are clearly demarcated from the surrounding dermis histologically lacking the completely ordered collagen organization characteristic of normal skin. The collagen fibers go in multiple directions giving the scar stiffer biomechanical properties and are of variable width depending on the genetics of the patient and the underlying tension placed on the healing immature scar. There is no longer any increased fluid in the tissues (edema) and so the scar is flat. The color of the scar ranges from white (absence of melanocytes) to hyperpigmented (often characteristic of ethnicities with increased melanocytes in the basal layer of the epidermis such as Asian, South Asian, Middle Eastern, Mediterranean, and Latin American or patients with brown skin).
3 Atrophic Scar
In some cases, a scar will become depressed or thinned as it transitions from an immature scar (◘ Fig. 9.3). This can occur when collagen synthesis is depressed and inflammation is less than usual. Examples of atrophic scars are the stretch marks or striae that can be a complication of systemic steroid excess either from exogenous steroids or Cushing’s disease or in some scars after radiation therapy.
4 Linear Hypertrophic Scar
In many cases, scars fail to transition normally from immature to mature with resolution of inflammation and an equilibrium of collagen synthesis and breakdown. Collagen continues to accumulate, and the scar widens and becomes elevated or ropy in appearance and the erythema fails to resolve. This active process of scar growth can continue for many months but eventually slowly resolves, with resolution of erythema that can take years. The collagen accumulation stabilizes but with a residual scar that is elevated and wider than a normal mature scar (◘ Fig. 9.4). During the period of active collagen accumulation and erythema, the scar can be itchy and/or painful. The residual scar is less elastic (stiffer) than normal skin and, if it crosses a joint, can limit motion. A key feature of hypertrophic scar (versus keloid) is that the scar tissue remains within the confines of the original scar (although it may be widened). Hypertrophic scars are more common in patients of color, particularly East Asians, and the susceptibility to hypertrophic scars is often inherited. Prolonged inflammation for any reason (delays in epithelization, blocked sebaceous glands, ingrown hairs, and tension) contributes significantly to the incidence of hypertrophic scars. Frequently portions of a scar will be hypertrophic in hair-bearing areas, while the adjacent scars evolve into normal mature scars.
5 Widespread Hypertrophic Scar
In many cases, most notably from burns, the initial injury is not linear but covers a larger area (◘ Fig. 9.5). In general, when epithelization (complete epithelial resurfacing) is not completed within 2 weeks, the risk of hypertrophic scar increases, particularly in children and in adults under the age of 40. In a hypertrophic scar , the period of collagen accumulation is prolonged up to 6–12 months resulting in a scar that is elevated and thickened and is very stiff. Erythema will be prolonged and the scars are often intensely pruritic or even painful.
6 Keloid
A keloid unlike a hypertrophic scar behaves more like a tumor in that growth can occur even years after the original injury and extend far beyond the confines of the original scar. The keloid often has a mushroom or cauliflower type of appearance. Keloids are frequently symptomatic with pain or itch. The genetics of keloids are quite complex and beyond the scope of this chapter, but many are familial and are much more common among many African tribes and to a lesser degree among Asians. The central portion of a keloid is very dense fibers with a characteristic pattern histologically and relatively acellular, while the active spreading edge has a significant inflammatory and cellular component.
6.1 Minor Keloid
Most keloids arise from localized injuries such as a pierced ear (the most common location of minor keloid) , and although they extend beyond the margins of the original scar, their growth stabilizes and allows more options for treatment including surgical excision combined with other modalities such as steroid injections or radiation therapy (◘ Fig. 9.6).
6.2 Major Keloid
In extreme situations in patients with a very strong genetic predisposition, keloids can extend into large plagues with a very actively growing outer edge that in some areas has a characteristic butterfly pattern (◘ Fig. 9.7a) due to forces on the keloid exerted by the surrounding skin. The peripheral edge of the keloid is active, while the central portion is less active with less cell proliferation. Often the original injury can be as minor as a scratch or seemingly spontaneous (◘ Fig. 9.7b Keloid arising from small tracheotomy scar), and the unfortunate patients with major keloids can form them all over their body. These are extraordinarily difficult to treat and are both deforming and debilitating. Intensive research on the defining genetic characteristics and pathogenesis of keloids continues, and in a few cases genetic loci have been identified, but the genetics are complex, as well as the pathogenesis.
Bibliography
Mustoe TA, Cooter R, Gold M, Hobbs R, Ramelet AA, Shakespeare P, Stella M, Teot L, Wood F, Ziegler U. International clinical guidelines for scar management. Plast Reconstr Surg. 2002;110:560–72.
Kim S, Choi TH, Liu W, Ogawa R, Mustoe TA. Update on scar management: guidelines for treating Aisian patients. Plast Reconstr Surg. 2013;132:1580–9.
Gold MH, Berman B, Clementoni MT, Gauglitz GG, Nahai F, McGuire M, Mustoe TA, Pusic A, Sachdev M, Teo L, Waibel J. International clinical recommendations on scar management: part 1 – evaluating the evidence and updated international clinical recommendations on scar management: part 2 – algorithms for scar prevention and treatment. Dermatol Surg. 2014;40(8):825–31.
Bartell TH, Monafo WW, Mustoe TA. Noninvasive in vivo quantification of elastic properties of hypertrophic scar: hand held elastometry. J Burn Care Rehabil. 1988;9:657–60.
Widgerow AD, Chait LA. Scar management practice and science: a comprehensive approach to controlling scar tissue and avoiding hypertrophic scarring. Adv Skin Wound Care. 2011;24(12):555–61. (meta-analysis).
Al-Attar A, Mess S, Thomassen JM, et al. Keloid pathogenesis and treatment. Plast Reconstr Surg. 2006;117(1):286–300.
Wolfram D, Tzankov A, Pulzi P, Piza-Katzer H. A review of hypertrophic scars and keloids-their pathophysiology, risk factors and management. Dermatol Surg. 2009;35:171–81.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2020 The Author(s)
About this chapter
Cite this chapter
Mustoe, T.A. (2020). International Scar Classification in 2019. In: Téot, L., Mustoe, T.A., Middelkoop, E., Gauglitz, G.G. (eds) Textbook on Scar Management. Springer, Cham. https://doi.org/10.1007/978-3-030-44766-3_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-44766-3_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-44765-6
Online ISBN: 978-3-030-44766-3
eBook Packages: MedicineMedicine (R0)