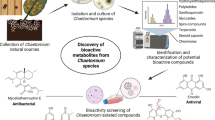
Abstract
Chaetomium is one of the largest genera of saprobic ascomycetes with more than 300 species worldwide; the most common species are C. globosum and C. elatum. Many species in this genus are well known to produce a wide range of natural products; most of the products have a wide spectrum of biological activities, whilst some are toxigenic. Currently, there are more than 300 known bioactive metabolites produced by different Chaetomium spp. In this chapter, the most biologically important toxic metabolites of a variety of Chaetomium species are discussed. Secondary metabolites from Chaetomium have potential biotechnological applications with a market value beyond those in human health, medicine, industrial production, food, or agricultural industries.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Abraham WR, Arfmann HA (1992) Rearranged tetrahydrofurans from Chaetomium cochlioides. Phytochemistry 31:2405–2408
Aly AH, Debbab A, Edrada-Ebel R, Wray V, Müller WE, Lin W, Ebel R, Proksch P (2009) A new tetrahydrofuran derivative from the endophytic fungus Chaetomium sp. isolated from Otanthus maritimus. Z Naturforsch C 64:350–354
Ariza MR, Larsen TO, Peterson BO, Duus JO, Barrero AF (2002) Penicillium digitatum metabolites on synthetic media and citrus fruits. J Agric Food Chem 50:6361–6365
Baliah V, Jeyaraman R, Chandrasekaran L (1983) Synthesis of 2,6-disubstituted piperidines, oxanes, and thianes. Chem Rev 83:379–423
Beisswenger PJ, Howell SK, Smith K, Szwergold BS (2003) Glyceraldehyde-3-phosphate dehydrogenase activity as an independent modifier of methylglyoxal levels in diabetes. Biochem Biophys Acta 1637:98–106
Bitzer J (2005) Isolierung und Strukturaufklärung neuer Naturstoffe aus Bakterien und endophytischen Pilzen durch chemisches screening. Dissertation, University of Göttingen
Bohlmann F, Ziesche J (1979) Ein ungewöhnliches tetrahydrofuranderivat aus Helichrysum aureo-nitens. Phytochemistry 18:664–665
Brewer D, Jerram W, Taylor A (1968) The production of cochliodinol and a related metabolite by Chaetomium species. Can J Microbiol 14:861–866
Brewer D, Jerram WA, Meiler D, Taylor A (1970) The toxicity of cochliodinol, an antibiotic metabolite of Chaetomium spp. Can J Microbiol 16:433–440
Burrows BF (1967) A new fungal tetrahydrofuran. J Chem Soc Chem Commun 12:597–598
Cole RJ, Kirksey JW, Cutler HG, Davis EE (1974) Toxic effects of oosporein from Chaetomium trilaterale. J Agric Food Chem 22:517–520
Cutler HG (1984) Biologically active natural products from fungi: templates for tomorrow’s pesticides. In: Ory RL, Rittig FR (eds) Bioregulators, chemistry and uses. American Chemical Society, Washington, DC, pp 153–170
Demain AL, Fang A (2000) The natural functions of secondary metabolites. In: Fiechter A (ed) History of modern biotechnology I, advances in biochemical engineering/biotechnology, vol 69. Springer, Berlin, pp 1–39. https://doi.org/10.1007/3-540-44964-7_1
Dias DA, Urban S, Roessner U (2012) A historical overview of natural products in drug discovery. Meta 2:303–336
Ding G, Song YC, Chen JR, Xu C, Ge HM, Wang XT, Tan RX (2006) Chaetoglobosin U, a cytochalasan alkaloid from endophytic Chaetomium globosum IFB-E019. J Nat Prod 69:302–304
Dong JY, He HP, Shen YM, Zhang KQ (2005) Nematicidal epipolysulfanyldioxopiperazines from Gliocladium roseum. J Nat Prod 68:1510–1513
Fox EM, Howlett BJ (2008) Secondary metabolism: regulation and role in fungal biology. Curr Opin Microbiol 11:481–487
Fujimoto H, Nozawa M, Okuyama E, Ishibashi M (2003) Six new constituents from an ascomycete, Chaetomium quadrangulatum, found in a screening study focused on monoamine oxidase inhibitory activity. Chem Pharm Bull 51(3):247–251
Fujimoto H, Sumino M, Okuyama E, Ishibashi M (2004) Immunomodulatory constituents from an ascomycete, Chaetomium seminudum. J Nat Prod 67:98–102
Gardiner DM, Waring P, Howlett B (2005) The epipolythiodioxopiperazine (ETP) class of fungal toxins: distribution, mode of action, functions and biosynthesis. J Microbiol 151:1021–1032
Ge HM, Zhang WY, Ding G, Saparpakorn P, Song YC, Hannongbua S, Tan RX (2008) Chaetoglobins A and B, two unusual alkaloids from endophytic Chaetomium globosum culture. Chem Commun:5978–5980
Ge HM, Zhang Q, Xu SH, Guo ZK, Song YC, Huang WY, Tan RX (2011) Chaetoglocins A-D, four new metabolites from the endophytic fungus Chaetomium globosum. Planta Med 77:277–280
Geiger WB (1949) Chetomin, an antibiotic substance from Chaetomium cochliodes III. Composition and functional groups. Arch Biochem 21:125–131
Gloer JB (2007) Applications of fungal ecology in the search for new bioactive natural products. In: Kubicek CP, Druzhinina IS (eds) The mycota, vol IV, 2nd edn. Springer-Verlag, New York
Gurnani N, Mehta D, Gupta M, Mehta BK (2014) Natural products: source of potential drugs. Afr J Basic Appl Sci 6:171–186
Hauser D, Zardin T (1972) Isolation of 6-hydroxymethyleugenin from Chaetomium minutum. Experientia 28:1114
Hauser D, Weber HP, Sigg HP (1970) Isolation and configuration of chaetocin. Helv Chim Acta 53:1061–1073
He L, Zhong-Bin L, Dan T, Wen-Bo H, Qiang Z, Jin-Ming G (2018) Polyketides from two Chaetomium species and their biological functions. J Antibiot 71:677–681. https://doi.org/10.1038/s41429-018-0047-x
Hwang EI, Yun BS, Kim YK, Kwon BM, Kim HG, Lee HB, Bae KS, Kim SU (2000) Chaetoatrosin A, a novel chitin synthase II inhibitor produced by Chaetomium atrobrunneum F449. J Antibiot 53:248–255
Itoh Y, Kodama K, Furuya K, Takahashi S, Haneishi T, Takiguchi Y, Arai M (1980) A new sesquiterpene antibiotic, heptelidic acid producing organisms, fermentation, isolation and characterization. J Antibiot 33(5):468–473
Jiao W, Feng Y, Blunt JW, Cole AL, Munro MH (2004) Chaetoglobosins Q, R, and T, three further new metabolites from Chaetomium globosum. J Nat Prod 67:1722–1725
Kanokmedhakul S, Kanokmedhakul K, Phonkerd N, Soytong K, Kongsaeree P, Suksamrarn A (2002) Antimycobacterial anthraquinone-chromanone compound and diketopiperazine alkaloid from the fungus Chaetomium globosum KMITL-N0802. Planta Med 68:834–836
Kawagishi H, Katsumi R, Sazawa T, Mizuno T, Hagiwara T, Nakamura T (1988) Cytotoxic steroids from the mushroom Agaricus blazei. Phytochemistry 27:2777–2779
Keller NP, Turner G, Bennett JW (2005) Fungal secondary metabolism – from biochemistry to genomics. Nat Rev Microbiol 3:937–947. https://doi.org/10.1038/nrmicro1286
Keyang L, Yisheng Z, Li L, Xuewei W, Gang D (2013) Chaetochromones A and B, two new polyketides from the fungus Chaetomium indicum (CBS.860.68). Molecules 18:10944–10952. https://doi.org/10.3390/molecules180910944
Khumkomkhet P, Kanokmedhakul S, Kanokmedhakul K, Hahnvajanawong C, Soytong K (2009) Antimalarial and cytotoxic depsidones from the fungus Chaetomium brasiliense. J Nat Prod 72:1487–1491
Kikuchi T, Kadota S, Suehara H, Nishi A, Tubaki K (1981) Odorous metabolites of a fungus, Chaetomium globosum Kinze ex Fr. Identification of geosmin, a musty-smelling compound. Chem Pharm Bull 29:1782–1784
Kim JH, Lee CH (2009) Heptelidic acid, a sesquiterpene lactone, inhibits etoposide-induced apoptosis in human leukemia U937 cells. J Microbiol Biotechnol 19:787–791
Kingsland SR, Barrow RA (2009) Identification of chaetoviridin E from a cultured microfungus, Chaetomium sp. and structural reassignment of chaetoviridins B and D. Aust J Chem 62:269–274
Koyama K, Akiba M, Imaizumi T, Kinoshita K, Takahashi K, Suzuki A, Yano S, Horie S, Watanabe K (2002) Antinociceptive constituents of Auricularia polytricha. Planta Med 68:284–285
Kubohara Y, Okamoto K, Tanaka Y, Asahi K, Sakurai A, Takahashi N (1993) Differanisole A, an inducer of the differentiation of friend leukemic cells, induces stalk cell differentiation in Dictyostelium discoideum. FEBS Lett 322:73–75
Kumagai S, Narasaki R, Hasumi K (2008) Glucose-dependent active ATP depletion by koningic acid kills high-glycolytic cells. Biochem Biophys Res Commun 365:362–368
Li GY, Li BG, Yang T, Yan JF, Liu GY, Zhang GL (2006) Chaetocochins A-C, epipolythiodioxopiperazines from Chaetomium cochliodes. J Nat Prod 69:1374–1376
Li GY, Li BG, Yang T, Liu GY, Zhang GL (2008) Secondary metabolites from the fungus Chaetomium brasiliense. Helv Chim Acta 91:124–129
Li LM, Zou Q, Li GY (2010) Chromones from an ascomycete, Chaetomium aureus. Chin Chem Lett 21:1203–1205
Lloyd G, Robertson A, Sankey GB, Whalley WB (1955) The chemistry of fungi. Part XXV. Oosporein, a metabolite of Chaetomium aureum chivers. J Chem Soc:2163–2165
Lösgen S, Schlörke O, Meindl K, Herbst-Irmer R, Zeeck A (2007) Structure and biosynthesis of chaetocyclinones, new polyketides produced by an endosymbiotic fungus. Eur J Org Chem 2007(13):2191–2196
Manning RO, Wyatt RD (1984) Comparative toxicity of Chaetomium contaminated corn and various chemical forms of oosporein in broiler chicks. Poult Sci 63:251–259
Marwah RG, Fatope MO, Deadman ML, Al-Maqbali YM, Husband J (2007) Musanahol: a new aureonitol-related metabolite from a Chaetomium sp. Tetrahedron 63:8174–8180
Mason SF, Vane GW (1967) The circular dichroisrn and the stereochemistry of a new fungal tetrahydrofuran. J Chem Soc Chem Commun (12):598
McInnes AG, Taylor A, Walter JA (1976) The structure of chetomin. J Am Chem Soc 98:6741–6741
Meiler D, Taylor A (1971) The effect of cochliodinol, a metabolite of Chaetomium cochliodes, on the respiration of microsopores of Fusarium oxysporum. Can J Microbiol 17:83–86
Müllbacher A, Waring P, Tiwari-Palni U, Eichner RD (1986) Structural relationship of epipolythiodioxopiperazines and their immunomodulating activity. Mol Immunol 23:231–235
Muroga Y, Yamada T, Numata A, Tanaka R (2009) Chaetomugilins I-O, new potent cytotoxic metabolites from a marine-fish derived Chaetomium species. Stereochemistry and biological activities. Tetrahedron 65:7580–7586
Nagaoka T, Nakata K, Kouno K, Ando T (2004) Antifungal activity of oosporein from an antagonistic fungus against Phytophthora infestans. Z Naturforsch C 59:302–304
Nakao Y, Kuo J, Yoshida WY, Kelly M, Scheuer PJ (2003) More kapakahines from the marine sponge Cribrochalina olemda. Org Lett 5:1387–1390
Nakazawa M, Uehara T, Nomura Y (1997) Koningic acid (a potent glyceraldehyde-3-phosphate dehydrogenase inhibitor)-induced fragmentation and condensation of DNA in NG108-15 cells. J Neurochem 68:2493–2499
Nielsen KF, Gravesen S, Nielsen PA, Andersen B, Thrane U, Frisvad JC (1999) Production of mycotoxins on artificially and naturally infested building materials. Mycopathologia 145:43–56
Oh H, Swenson DC, Gloer JB, Wicklow DT, Dowd PF (1998) Chaetochalasin A: a new bioactive metabolite from Chaetomium brasiliense. Tetrahedron Lett 39:7633–7636
Oka H, Asahi K, Morishima H, Sanada M, Shiratori K, Iimura Y, Sakurai T, Uzawa J, Iwadare S, Takahashi N (1985) Differanisole A, a new differentiation inducing substance. J Antibiot 38:1100–1102
Phonkerd N, Kanokmedhakul S, Kanokmedhakul K, Soytong K, Prabpai S, Kongsearee P (2008) Bis-spiro-azaphilones and azaphilones from the fungi Chaetomium cochliodes VTh01 and C. cochliodes CTh05. Tetrahedron 64:9636–9645
Pontius A, Krick A, Kehraus S, Brun R, König GM (2008) Antiprotozoal activities of heterocyclic-substituted xanthones from the marine-derived fungus Chaetomium sp. J Nat Prod 71:1579–1584
Powell JW, Whalley WB (1969) The chemistry of fungi. 58. Structures of colletodiol, a metabolite of Chaetomium funicola. J Chem Soc Perkin Trans 1:911–912
Qin JC, Gao JM, Zhang YM, Yang SX, Bai MS, Ma YT, Laatsch H (2009) Polyhydroxylated steroids from an endophytic fungus, Chaetomium globosum ZY-22 isolated from Ginkgo biloba. Steroids 74:786–790
Rether J, Erkel G, Anke T, Sterner O (2004) Inhibition of inducible TNF-alpha expression by oxaspirodion, a novel spiro-compound from the ascomycete Chaetomium subspirale. Biol Chem 385:829–834
Royles BJL (1995) Naturally occurring tetramic acids: structure, isolation, and synthesis. Chem Rev 95:1981–2001
Rui HJ, Shu X, Jun YL, Hui MG, Hui D, Chen X, Hai LZ, Ren XT (2006) Chaetominine, a cytotoxic alkaloid produced by endophytic Chaetomium sp. IFB-E015. Org Lett 8(25):5709–5712
Safe S, Taylor A (1972) Sporidesmins. Part XIII. Ovine 111-thrift in Nova Scotia. Part 11. The characterisation of chetomin, a toxic metabolite of Chaetomium cochliodes and Chaetomium globosum. J Chem Soc Perkin Trans 1:472–479
Saito M, Seto H, Yonehara H (1983) Biosynthesis of 2-(but-l,3-dienyl)-3-hydro-4-(penta-l,3-dienyl)-tetrahydrofuran, a metabolite of Chaetomium coarctatum. Agric Biol Chem 47:2935–2937
Saito T, Suzuki Y, Koyama K, Natori S, Iitaka Y, Kinoshita T (1985) Chetracin A and chaetocins B and C, three new epipolythiodioxopiperazines from Chaetomium spp. Chem 26:4731–4734
Saito T, Koyama K, Natori S, Iitaka Y (1988) Chetracin A, a new epipolythiodioxopiperazine having a tetrasulfide bridge from Chaetomium abuens and C. retardatum. Tetrahedron Lett Pharm Bull 36:1942–1956
Schlörke O, Zeeck A (2006) Orsellides A–E: an example for 6-deoxyhexose derivatives produced by fungi. Eur J Org Chem 2006(4):1043–1049. https://doi.org/10.1002/ejoc.200500793
Sekita S (1983) Isocochliodinol and neocochliodinol, bis(3-indolyl)-benzoquinones from Chaetomium spp. Chem Pharm Bull 31:2998–3001
Sekita S, Yoshihira K, Natori S, Udagawa S, Muroi T, Sugiyama Y, Kurata H, Umeda M (1981) Mycotoxin production by Chaetomium spp. and related fungi. Can J Microbiol 27:766–772
Seto H, Saito M, Uzawa J, Yonehara H (1979) Utilization of 13C -13C coupling in structural and biosynthetic studies. XII. biosynthesis of 2-(but-1,3-dienyl)-3-hydro-4-(penta-1,3-dienyl)-tetrahydrofuran, A metabolite of Chaetomium coarctaum. Heterocycles 13:247–253
Smetanina OF, Kuznetzova TA, Denisenko VA, Pivkin MV, Khudyakova YV, Gerasimenko AV, Popov DY, Il’in SG, Elyakov GB (2001) 3β-Methoxyolean-18-ene (miliacin) from the marine fungus Chaetomium olivaceum. Russ Chem Bull 50:2463–2465
Stadler M, Keller NP (2008) Paradigm shifts in fungal secondary metabolite research. Mycol Res 112(2):127–130
Sutton DA, Fothergill A, Rinaldi MG (1998) Guide to clinically significant fungi. Williams and Wilkins, Baltimore
Suzuki T, Oka H, Okura A, Asahi K, Takahashi N (1986) In vitro and in vivo effects of differanisole A on some tumor cells. J Antibiot 39:869–871
Takahashi M, Koyama K, Natori S (1990) Four new azaphilones from Chaetomium globosum var. flavo-viridae. Chem Pharm Bull 38:625–628
Tanaka Y, Shiomi K, Kamei K, Sugoh-Hagino M, Enomoto Y, Fang F, Yamaguchi Y, Masuma R, Zhang CG, Zhang XW, Omura S (1998) Antimalarial activity of radicicol, heptelidic acid and other fungal metabolites. J Antibiot 51:153–160
Teigo A, Shuntaro M, Naoki S, Tohru T, Kenji M, Hiroaki S, Tomoji O, Yoshiteru O (2012) Structural diversity of new c13-polyketides produced by Chaetomium mollipilium cultivated in the presence of a nad þ-dependent histone deacetylase inhibitor. Org Lett 14(21):5456–5459
Terry BJ, Liu WC, Cianci CW, Proszynski E, Fernandes P, Bush K, Meyers E (1992) Inhibition of herpes simplex virus type 1 DNA polymerase by the natural product oosporein. J Antibiot 45:286–288
Thohinung S, Kanokmedhakul S, Kanokmedhakul K, Kukongviriyapan V, Tusskorn O, Soytong K (2010) Cytotoxic 10-(indol-3-yl)-[13]cytochalasans from the fungus Chaetomium elatum ChE01. Arch Pharm Res 33:1135–1141
Tibodeau JD, Benson LM, Isham CR, Owen WG, Bible KC (2009) The anticancer agent chaetocin is a competitive substrate and inhibitor of thioredoxin reductase. Antioxid Redox Signal 11:1097–1106
Turbyville TJ, Wijeratne EM, Liu MX, Burns AM, Seliga CJ, Luevano LA, David CL, Faeth SH, Whitesell L, Gunatilaka AAL (2006) Search for Hsp90 inhibitors with potential anticancer activity: isolation and SAR studies of radicicol and monocillin I from two plant-associated fungi of the Sonoran desert. J Nat Prod 69:178–184
Udagawa S, Muroi T, Kurata H, Sekita S, Yoshihira K, Natori S, Umeda M (1979) The production of chaetoglobosins, sterigmatocystin, O-methylsterigmatocystin, and chaetocin by Chaetomium spp. and related fungi. Can J Microbiol 25:170–177
Wang S, Li XM, Teuscher F, Li DL, Diesel A, Ebel R, Proksch P, Wang BG (2006) Chaetopyranin, a benzaldehyde derivative, and other related metabolites from Chaetomium globosum, an endophytic fungus derived from the marine red alga Polysiphonia urceolata. J Nat Prod 69:1622–1625
Wang S, Xu Y, Maine EA, Wijeratne EM, Espinosa-Artiles P, Gunatilaka AA, Molnár I (2008) Functional characterization of the biosynthesis of radicicol, an Hsp90 inhibitor resorcylic acid lactone from Chaetomium chiversii. Chem Biol 15:1328–1338
Weindling R, Emerson OH (1936) The isolation of a toxic substance from the culture filtrate of Trichoderma. Phytopathology 26:1068–1070
Wijeratne EMK, Turbyville TJ, Fritz A, Whitesell L, Gunatilaka AAL (2006) A new dihydroxanthenone from a plant associated strain of the fungus Chaetomium globosum demonstrates anticancer activity. Bioorg Med Chem 14:7917–7923
Wu ZJ, Li GY, Fang DM, Qi HY, Ren WJ, Zhang GL (2008) Analysis of epipolythiodioxopiperazines in fungus Chaetomium cochliodes using HPLC-ESI-MS/MS/MS. Anal Chem 80:217–226
Xu GB, Li LM, Yang T, Zhang GL, Li GY (2012) Chaetoconvosins A and B, alkaloids with new skeleton from fungus Chaetomium convolutum. Org Lett 14(23):6025–6055
Yamada T, Muroga Y, Tanaka R (2009) New azaphilones, secochaetomugilins A and D, produced by a marine-fish-derived Chaetomium globosum. Mar Drugs 7:249–257
Yamazaki M, Fujimoto H, Okuyama E (1978) Structure determination of six fungal metabolites, tryptoquivaline E, F, G, H, I and J from Aspergillus fumigatus. Chem Pharm Bull 26:111–117
Yamori T, Matsunaga A, Sato S, Yamazaki K, Komi A, Ishizu K, Mita I, Edatsugi H, Matsuba Y, Takezawa K, Nakanishi O, Kohno H, Nakajima Y, Komatsu H, Andoh T, Tsuruo T (1999) Potent antitumor activity of MS-247, a novel DNA minor groove binder, evaluated by an in vitro and in vivo human cancer cell line panel. Cancer Res 59:4042–4049
Yang SX, Gao JM, Zhang Q, Laatsch H (2011) Toxic polyketides produced by Fusarium sp., an endophytic fungus isolated from Melia azedarach. Bioorg Med Chem Lett 21:1887–1889
Yeung BKS, Nakao Y, Kinnel RB, Carney JR, Yoshida WY, Scheuer PJ, Kelly-Borges M (1996) The kapakahines, cyclic peptides from the marine sponge Cribrochalina olemda. J Org Chem 61:7168–7173
Zhang J, Ge HM, Jiao RH, Li J, Peng H, Wang YR, Wu JH, Song YC, Tan RX (2010) Cytotoxic chaetoglobosins from the endophyte Chaetomium globosum. Planta Med 76:1910–1914
Zhang Q, Li HQ, Zong SC, Gao JM, Zhang AL (2012) Chemical and bioactive diversities of the genus Chaetomium secondary metabolites. Mini Rev Med Chem 12(2):127–148
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Hamed, S.R., Abdel-Azeem, A.M., Dar, P.M. (2020). Recent Advancements on the Role of Biologically Active Secondary Metabolites from Chaetomium. In: Abdel-Azeem, A. (eds) Recent Developments on Genus Chaetomium . Fungal Biology. Springer, Cham. https://doi.org/10.1007/978-3-030-31612-9_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-31612-9_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-31611-2
Online ISBN: 978-3-030-31612-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)