Abstract
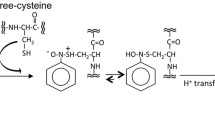
Biological reactive intermediates can be created via metabolism of xenobiotics during the process of chemical elimination. They can also be formed as by-products of cellular metabolism, which produces reactive oxygen and nitrogen species. These reactive intermediates tend to be electrophilic in nature, which enables them to interact with tissue macromolecules, disrupting cellular signaling processes and often producing acute and chronic toxicities. Quinones are a well-known class of electrophilic species. Many natural products contain quinones as active constituents, and the quinone moiety exists in a number of chemotherapeutic agents. Quinones are also frequently formed as electrophilic metabolites from a variety of xeno- and endobiotics. Hydroquinone (HQ) is present in the environment from various sources, and it is also a known metabolite of benzene. HQ is converted in the body to 1,4-benzoquinone, which subsequently gives rise to hematotoxic and nephrotoxic quinone–thioether metabolites. The toxicity of these metabolites is dependent upon their ability to arylate proteins and to produce oxidative stress. Protein tertiary structure and protein amino acid sequence combine to determine which proteins are targets of these electrophilic quinone–thioether metabolites. We have used cytochrome c and model peptides to view adduction profiles of quinone–thioether metabolites, and have determined by MALDI-TOF analysis that these electrophiles target specific residues within these model systems.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Monks, T.J. (2006) Introduction. Drug Metab Rev 38, 599–600.
Brouwers, O., Teerlink, T., van Bezu, J., Barto, R., Stehouwer, C., and Schalkwijk, C. (2007) Methylglyoxal and methylglyoxal-arginine adducts do not directly inhibit endothelial nitric oxide synthase. Ann. N. Y. Acad. Sci. 1126, 231–234.
Kankova, K. (2008) Diabetic threesome (hyperglycaemia, renal function and nutrition) and advanced glycation end products: evidence for the multiple-hit agent?, Proc. Nutr. Soc. 67, 60–74.
Carbone, D.L., Doorn, J.A., Kiebler, Z., and Petersen, D.R. (2005) Cysteine modification by lipid peroxidation products inhibits protein disulfide isomerase. Chem. Res. Toxicol. 18, 1324–1331.
Sampey, B.P., Carbone, D.L., Doorn, J.A., Drechsel, D.A., and Petersen, D.R. (2007) 4-Hydroxy-2-nonenal adduction of extracellular signal-regulated kinase (Erk) and the inhibition of hepatocyte Erk-Est-like protein-1-activating protein-1 signal transduction. Mol. Pharmacol. 71, 871–883.
Anderson, W.B., Board, P.G., and Anders, M.W. (2004) Glutathione transferase zeta-catalyzed bioactivation of dichloroacetic acid: reaction of glyoxylate with amino acid nucleophiles. Chem. Res. Toxicol. 17, 650–662.
Go, Y.M., Halvey, P.J., Hansen, J.M., Reed, M., Pohl, J., and Jones, D.P. (2007) Reactive aldehyde modification of thioredoxin-1 activates early steps of inflammation and cell adhesion. Am. J. Pathol. 171, 1670–1681.
Luo, J., Hill, B.G., Gu, Y., Cai, J., Srivastava, S., Bhatnagar, A., and Prabhu, S.D. (2007) Mechanisms of acrolein-induced myocardial dysfunction: implications for environmental and endogenous aldehyde exposure. Am. J. Physiol. Heart. Circ. Physiol. 293, H3673–3684.
Baillie, T.A. (2006) Future of toxicology-metabolic activation and drug design: challenges and opportunities in chemical toxicology. Chem. Res. Toxicol. 19, 889–893.
Ross, D. (2000) The role of metabolism and specific metabolites in benzene-induced toxicity: evidence and issues. J. Toxicol. Environ. Health A 61, 357–372.
Pagano, G. (2002) Redox-modulated xenobiotic action and ROS formation: a mirror or a window? Hum. Exp. Toxicol. 21, 77–81.
Bolton, J.L., Trush, M.A., Penning, T.M., Dryhurst, G., and Monks, T.J. (2000) Role of quinones in toxicology. Chem. Res. Toxicol. 13, 135–160.
Verrax, J., Delvaux, M., Beghein, N., Taper, H., Gallez, B., and Buc Calderon, P. (2005) Enhancement of quinone redox cycling by ascorbate induces a caspase-3 independent cell death in human leukaemia cells. An in vitro comparative study. Free Radic. Res. 39, 649–657.
Ruiz-Ramos, R., Cebrian, M.E., and Garrido, E. (2005) Benzoquinone activates the ERK/MAPK signaling pathway via ROS production in HL-60 cells. Toxicology 209, 279–287.
Person, M.D., Mason, D.E., Liebler, D.C., Monks, T.J., and Lau, S.S. (2005) Alkylation of cytochrome c by (glutathion-S-yl)-1,4-benzoquinone and iodoacetamide demonstrates compound-dependent site specificity. Chem. Res. Toxicol. 18, 41–50.
Lindsey, R.H., Jr., Bender, R.P., and Osheroff, N. (2005) Effects of benzene metabolites on DNA cleavage mediated by human topoisomerase II alpha: 1,4-hydroquinone is a topoisomerase II poison. Chem. Res. Toxicol. 18, 761–770.
Lau, S.S., Hill, B.A., Highet, R.J., and Monks, T.J. (1988) Sequential oxidation and glutathione addition to 1,4-benzoquinone: correlation of toxicity with increased glutathione substitution. Mol. Pharmacol. 34, 829–836.
Peters, M.M., Jones, T.W., Monks, T.J., and Lau, S.S. (1997) Cytotoxicity and cell-proliferation induced by the nephrocarcinogen hydroquinone and its nephrotoxic metabolite 2,3,5-(tris-glutathion-S-yl)hydroquinone. Carcinogenesis 18, 2393–2401.
Kleiner, H.E., Jones, T.W., Monks, T.J., and Lau, S.S. (1998) Immunochemical analysis of quinol–thioether-derived covalent protein adducts in rodent species sensitive and resistant to quinol–thioether-mediated nephrotoxicity. Chem. Res. Toxicol. 11, 1291–1300.
Yoon, H.S., Monks, T.J., Walker, C.L., and Lau, S.S. (2001) Transformation of kidney epithelial cells by a quinol thioether via inactivation of the tuberous sclerosis-2 tumor suppressor gene. Mol. Carcinog. 31, 37–45.
Kussmann, M., Lassing, U., Sturmer, C.A., Przybylski, M., and Roepstorff, P. (1997) Matrix-assisted laser desorption/ionization mass spectrometric peptide mapping of the neural cell adhesion protein neurolin purified by sodium dodecyl sulfate polyacrylamide gel electrophoresis or acidic precipitation. J. Mass Spectrom. 32, 483–493.
Voyager. (2004) Voyager Biospectrometry Workstation Training, Applied Biosystems, Forester City, CA.
Stewart, B.J., Doorn, J.A., and Petersen, D.R. (2007) Residue-specific adduction of tubulin by 4-hydroxynonenal and 4-oxononenal causes cross-linking and inhibits polymerization. Chem. Res. Toxicol. 20, 1111–1119.
Fisher, A.A., Labenski, M.T., Malladi, S., Gokhale, V., Bowen, M.E., Milleron, R.S., Bratton, S.B., Monks, T.J., and Lau, S.S. (2007) Quinone electrophiles selectively adduct “electrophile binding motifs” within cytochrome c. Biochemistry 46, 11090–11100.
Acknowledgments
This work was supported by GM070890 (SSL) and ES07091 (AAF). The authors acknowledge the support of the P30 ES06694 Southwest Environmental Health Sciences Center, in particular the Arizona Proteomics Consortium (APC). Special thanks go to Dr. George Tsaprailis, Director of the APC.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media, LLC
About this protocol
Cite this protocol
Fisher, A.A., Labenski, M.T., Monks, T.J., Lau, S.S. (2011). Utilization of MALDI-TOF to Determine Chemical-Protein Adduct Formation In Vitro . In: Gautier, JC. (eds) Drug Safety Evaluation. Methods in Molecular Biology, vol 691. Humana Press. https://doi.org/10.1007/978-1-60761-849-2_18
Download citation
DOI: https://doi.org/10.1007/978-1-60761-849-2_18
Published:
Publisher Name: Humana Press
Print ISBN: 978-1-60327-186-8
Online ISBN: 978-1-60761-849-2
eBook Packages: Springer Protocols