Abstract
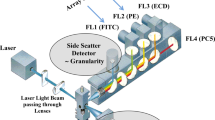
Flow cytometry is a powerful technique allowing multiparameter detection and quantification of single cells or particles including cell size, granularity, cell components (DNA, mRNA), surface receptors, intracellular proteins, and signaling events. The flow cytometer operates via three main systems: the fluidics, optics, and electronics, which work together to analyze the physical and chemical properties of your sample. The first system, the fluidics, transports your sample in a single stream through the instrument, from the sample tube, pass the lasers, and is either sorted for further experiments or discarded into the waste vessel. The second system, the optical system, is composed of a series of lasers; for excitation of your sample and attached fluorescence antibodies as it passes, a series of lenses; and a detector system. The third system is the electronic component, which enables the photocurrent from the detector system to be changed into electronic pulses to be processed by a computer and analyzed by flow cytometry software. Flow cytometry is thus a powerful technique, which is commonly used to determine the expression of cell surface markers and intracellular molecules to define cells into different populations by fluorescently labeled antibodies.
The staining procedure outlined below creates a single-cell suspension for staining with a panel of flow cytometry antibodies, which target different surface markers, to identify an array of cell types. After staining the sample is loaded into the flow cytometer, where the fluorescently labeled cells are excited as they pass by the laser emitting light at various wavelengths which are detected by the flow cytometer. Each fluorescent antibody has its own excitation and emission spectrum allowing the use of multiple antibodies. However, the emission spectrums of different fluorochromes can overlap each other, called spectral overlap. Thus, it is important to have good compensation controls to eliminate any antibody spillover.
The staining methods from this technique can be used for different cell types by changing the surface marker targeted by the flow antibody. It is also important to use knowledge of the density of surface molecule for detection and brightness of fluorochrome to guide antibody selection and also to titrate all antibodies prior to use.
This chapter’s protocol has been designed specifically for detection of human CD8+ T cells defining the activation status of the cells by surface marker phenotyping.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Gulzar N, Copeland KF (2004) CD8+ T-cells: function and response to HIV infection. Curr HIV Res 2(1):23–37
Berg RE, Forman J (2006) The role of CD8 T cells in innate immunity and in antigen non-specific protection. Curr Opin Immunol 18(3):338–343
Barber DL, Wherry EJ, Ahmed R (2003) Cutting edge: rapid in vivo killing by memory CD8 T cells. J Immunol 171(1):27–31
Bohana-Kashtan O, Civin CI (2004) Fas ligand as a tool for immunosuppression and generation of immune tolerance. Stem Cells 22(6):908–924
Flynn JK, Dore GJ, Hellard M, Yeung B, Rawlinson WD, White PA et al (2011) Early IL-10 predominant responses are associated with progression to chronic hepatitis C virus infection in injecting drug users. J Viral Hepat 18(8):549–561
Cashin K, Paukovics G, Jakobsen MR, Ostergaard L, Churchill MJ, Gorry PR et al (2014) Differences in coreceptor specificity contribute to alternative tropism of HIV-1 subtype C for CD4 + T-cell subsets, including stem cell memory T-cells. Retrovirology 11(1):97
Flynn JK, Gorry PR (2014) Stem memory T cells (TSCM)—their role in cancer and HIV immunotherapies. Clin Transl Immunology 3:e20
Flynn JK, Paukovics G, Cashin K, Borm K, Ellett A, Roche M et al (2014) Quantifying susceptibility of CD4+ stem memory T-cells to infection by laboratory adapted and clinical HIV-1 strains. Viruses 6(2):709–726
Flynn JK, Paukovics G, Moore MS, Ellett A, Gray LR, Duncan R et al (2013) The magnitude of HIV-1 resistance to the CCR5 antagonist maraviroc may impart a differential alteration in HIV-1 tropism for macrophages and T-cell subsets. Virology 442(1):51–58
Mahnke YD, Brodie TM, Sallusto F, Roederer M, Lugli E (2013) The who's who of T-cell differentiation: human memory T-cell subsets. Eur J Immunol 43(11):2797–2809
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Flynn, J., Gorry, P. (2019). Flow Cytometry Analysis to Identify Human CD8+ T Cells. In: Kaneko, S. (eds) In Vitro Differentiation of T-Cells. Methods in Molecular Biology, vol 2048. Humana, New York, NY. https://doi.org/10.1007/978-1-4939-9728-2_1
Download citation
DOI: https://doi.org/10.1007/978-1-4939-9728-2_1
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-4939-9727-5
Online ISBN: 978-1-4939-9728-2
eBook Packages: Springer Protocols