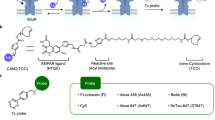
Abstract
Glutamate receptors (GluRs) located primarily on the membranes of neurons and glial cells are responsible for excitatory synaptic transmission in the central nervous system. The transport of GluRs to the cell surface is a highly regulated dynamic process that determines neuronal excitability and synaptic responses. The molecular and cellular mechanisms of GluR trafficking are often studied in cell cultures. These studies require sensitive techniques that allow the measurement of total and surface-expressed GluRs in cell populations. The cell-based enzyme-linked immunosorbent assay (cell-ELISA) combines steps of direct immunochemical labelling of cell cultures and ELISA. It can be used for quantitative comparisons of surface-expressed and total protein contents of various cell cultures. While several cell-ELISA protocols are available for different cell types, in this chapter we describe the procedure that we have applied for the investigation of quantitative changes in the cell surface expression of recombinant ionotropic glutamate receptors (iGluRs) in adherent human embryonic kidney 293 (HEK293) cells and endogenous iGluR proteins in primary neuronal cultures.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Molnár E (2018) Glutamate receptors. In: Choi S (ed) Encyclopedia of signaling molecules, 2nd edn. Springer, New York, pp 2138–2146
Noel J, Ralph GS, Pickard L, Williams J, Molnar E, Uney JB, Collingridge GL, Henley JM (1999) Surface expression of AMPA receptors in hippocampal neurons is regulated by an NSF-dependent mechanism. Neuron 23:365–376
Pickard L, Noël J, Henley JM, Collingridge GL, Molnár E (2000) Developmental changes in synaptic AMPA and NMDA receptor distribution and AMPA receptor subunit composition in living hippocampal neurons. J Neurosci 20:7922–7931
Pickard L, Noel J, Duckworth JK, Fitzjohn SM, Henley JM, Collingridge GL, Molnár E (2001) Transient synaptic activation of NMDA receptors leads to the insertion of native AMPA receptors into hippocampal neuronal plasma membrane. Neuropharmacology 41:700–713
Molnár E (2011) Long-term potentiation in cultured hippocampal neurons. Semin Cell Dev Biol 22:506–513
Rutter AR, Freeman FM, Stephenson FA (2002) Further characterization of the molecular interaction between PSD-95 and NMDA receptors: the effect of the NR1 splice variant and evidence for modulation of channel gating. J Neurochem 81:1298–1307
Papadakis M, Hawkins LM, Stephenson FA (2004) Appropriate NR1-NR1 disulfide-linked homodimer formation is requisite for efficient expression of functional, cell surface N-methyl-D-aspartate NR1/NR2 receptors. J Biol Chem 279:14703–14712
Cousins SL, Innocent N, Stephenson FA (2013) Neto1 associates with the NMDA receptor/amyloid precursor protein complex. J Neurochem 126:554–564
Horak M, Suh YH (2015) Counting NMDA receptors at the cell surface. NeuroMethods 106:31–44
Parnas D, Linial M (1998) Highly sensitive ELISA-based assay for quantifying protein levels in neuronal cultures. Brain Res Protocol 2:333–338
Lourenço EV, Roque-Barreira MC (2010) Immunoenzymatic quantitative analysis of antigens expressed on the cell surface (cell-ELISA). Methods Mol Biol 588:301–309
Hall RA, Soderling TR (1997) Quantitation of AMPA receptor surface expression in cultured hippocampal neurons. Neuroscience 78:361–371
Hall RA, Soderling TR (1997) Differential surface expression and phosphorylation of the N-methyl-D-aspartate receptor subunits NR1 and NR2 in cultured hippocampal neurons. J Biol Chem 272:4135–4140
Ball SM, Atlason PT, Shitu-Balogun OO, Molnár E (2010) Assembly and intracellular distribution of kainate receptors is determined by RNA editing and subunit composition. J Neurochem 114:1805–1818
Huh K-H, Wenthold RJ (1999) Turnover analysis of glutamate receptors identifies a rapidly degraded pool of the N-methyl-D-aspartate receptor subunit, NR1, in cultured cerebellar granule cells. J Biol Chem 274:151–157
Gallyas F, Ball S, Molnár E (2003) Assembly and cell surface expression of KA-2 subunit-containing kainate receptors. J Neurochem 86:1414–1427
Atlason PT, Scholefield CL, Eaves RJ, Mayo-Martin MB, Jane DE, Molnár E (2010) Mapping the ligand binding sites of kainate receptors: molecular determinants of subunit-selective binding of the antagonist [3H]UBP310. Mol Pharmacol 78:1036–1045
Gladding CM, Collett VJ, Jia Z, Bashir ZI, Collingridge GL, Molnár E (2009) Tyrosine dephosphorylation regulates AMPAR internalisation in mGluR-LTD. Mol Cell Neurosci 40:267–279
Molnár E (2013) Immunocytochemistry and immunohistochemistry. In: Langton PD (ed) Essential guide to reading biomedical papers: recognising and interpreting best practice. Wiley-Blackwell, Somerset, pp 117–128
Levite M (2014) Glutamate receptor antibodies in neurological diseases: anti-AMPA-GluR3 antibodies, anti-NMDA-NR1 antibodies, anti-NMDA-NR2A/B antibodies, anti-mGluR1 antibodies or anti-mGluR5 antibodies are present in subpopulations of patients with either: epilepsy, encephalitis, cerebellar ataxia, systemic lupus erythematosus (SLE) and neuropsychiatric SLE, Sjogren's syndrome, schizophrenia, mania or stroke. These autoimmune anti-glutamate receptor antibodies can bind neurons in few brain regions, activate glutamate receptors, decrease glutamate receptor's expression, impair glutamate-induced signaling and function, activate blood brain barrier endothelial cells, kill neurons, damage the brain, induce behavioral/psychiatric/cognitive abnormalities and ataxia in animal models, and can be removed or silenced in some patients by immunotherapy. J Neural Transm 121:1029–1075
Venkatesan A, Adatia K (2017) Anti-NMDA-receptor encephalitis: From bench to clinic. ACS Chem Neurosci 8:2586–2595
McIlhinney RA (2004) Generation and use of epitope-tagged receptors. Methods Mol Biol 259:81–98
Acknowledgment
This research was supported by grant from the Biotechnology and Biological Sciences Research Council, UK (Grant BB/J015938/1).
Conflict of Interest Disclosure: EM is a member of the Scientific Advisory Board of Hello Bio [www.hellobio.com].
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Molnár, E. (2019). Cell-Based Enzyme-Linked Immunosorbent Assay (Cell-ELISA) Analysis of Native and Recombinant Glutamate Receptors. In: Burger, C., Velardo, M. (eds) Glutamate Receptors. Methods in Molecular Biology, vol 1941. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-9077-1_4
Download citation
DOI: https://doi.org/10.1007/978-1-4939-9077-1_4
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-9076-4
Online ISBN: 978-1-4939-9077-1
eBook Packages: Springer Protocols