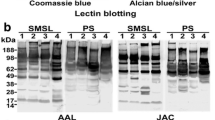
Abstract
Mucus is a complex exocrine secretion that covers the epithelial linings of higher animals. This secretion is derived from different types of epithelial glands which are composed of a variety of specialized cells. Consequently, tear, sputum, saliva, gastric juice, colonic and cervical mucus are all composed of a heterogeneous mixture of secretory products. Salivary mucus is produced by several glands (Table I). The bulk of it is derived from three main organ glands, namely the parotid which is serous in nature, the submandibular which contains both mucous and serous type acini, and the predominantly mucus secreting sublingual gland (1–5). In addition, numerous so-called minor salivary glands are dispersed throughout the oral soft tissue (6,7). These labial glands are composed mainly of mucous secreting cells, and consequently are a major source of the total mucins in saliva although they comprise only some 10% of the salivary volume produced daily. Mixed salivary secretions contain some 99.5% water; the remainder is made up of glycocon jugates, lipids, proteins, ions and small metabolites (8–12) . Due to its great variety of components, saliva plays multiple physiological roles.
The article is dedicated to Dr. Ward Pigman, one of the pioneers in the field of salivary mucin biochemistry, who died on september 30, 1977.
Abbreviations are listed in Appendix I entitled “A Guide for Carbohydrate Specificities of Lectins”.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
G. Quintarelli, Histochemical identification of salivary mucins. Ann. N.Y. Acad. Sci. 106:339–363 (1963).
J.M. Shakleford and W.H. Wilborn, Structural and histochemical diversity in mammalian salivary glands, Alabama J. Med. Sci. 5:180–203 (1968).
Banks, W.G., Applied Veterinary Histology. Williams Nilkins. Baltimore (1981).
Dellmanns, H.D., Veterinary Histology. Lea & Febiger, Philadelphia (1971).
G. Quintarelli, S. Tsuiki, Y. Hashimoto and W. Pigman, Studies of sialic acid-containing mucins in bovine submaxillary and rat sublingual glands. J. Histochem. Cytochem. 5:176–183 (1961).
L.R. Eversole, The histochemistry of mucosubstances in human minor salivary glands. Arch. Oral Biol. 2 7:1235–1239 (1972).
D.R. Green and G. Embery, Partial chemical characterization and biological activities of sulphated glycoproteins isolated from in vivo pilocarpine-stimulated secretions of rat minor salivary glands. Arch. Oral Biol. 29:859–863 (1984).
R.C. Caldwell and W. Pigman, Disc electrophoresis of human saliva in polyacrylamide gel. Arch. Biochem. Biophys. 110:91–96 (1965).
B.L. Slomiany, M. Aono, V.L.N. Murty, A. Slomiany, M.J. Levine and L.A. Tabak, Lipid composition of submandibular saliva from normal and cystic fibrosis individuals. J. Dent. Res. 61:1163–1166 (1982).
A.Bennick, Salivary acidic proline-rich proteins. Mol. Cell. Biochem. 45:83–99 (1982)
J.A. Young and C.A. Schneyer, Composition of saliva in mammalia. Australian J. Exp. Biol. Med. Sci.5.9: 1–53 (1981).
M. Mogi, B.Y. Hiraoka, K. Fukasawa, M. Harada, T. Kage and T. Ching, Two-dimensional electrophoresis in the analysis of a mixture of human sublingual and submandibular salivary proteins. Arch. Oral Biol.31:119–125 (1986).
A.P. Vreugdenhil, A.V. Nieuw Amerongen, G.L. Dange and P.A. Roukema, Localization of amylase and mucins in the major salivary glands of the mouse. Histochem. J. 14: 767–780 (1982).
M.S. Finkelstein, M. Tanner and M.L. Freedman, Salivary and serum I levels in a geriatric outpatient population. J. Clin. Immunol. 4:85–91 (1984).
M.R. Allansmith, J.L. Ebersole and C.A. Burns, I antibody levels in human tears, saliva and serum, Ann. N.Y. Acad. Sci. 409:166–168 (1983).
R.R. Arnold, M.F. Cole and J.R. Mhee, A bactericidal effect of human lactoferrin. Science 197:263–265 (1975).
J.D. Rudney, K.C. Kajander and Q.T. Smith, Correlation between human salivary levels of lysozyme, lactoferrin, salivary peroxidase and secretory immunoglobulin A with different stimulatory states and over time. Arch. Oral Biol. 30:765–771, (1985).
B. L. Lamberts, K.M. Pruitt, E.D. Pederson and M.P.Golding, Comparison of salivary peroxidase system components in caries-free and caries-active naval recruits. Caries Res. 25:488–494 (1984).
M.G. Humphreys-Behrer, Strain-specific differences in the proline-rich proteins and glycoproteins induced in rat salivary gland by chronic isoprenaline treatment. Biochem. J. 230:369–378, (1985).
M.N. Hatton, R.E. Loomis, M.J. Levine and L.A. Taback, Masticatory lubrication. Biochem. J. 230:817–820 (1985).
J.R. Clamp, The relationship between the immune system and mucus in the protection of mucous membranes. Biochem. Soc. Trans. 22:754–756 (1984).
P.A. Murray, M.J. Levine, L.A. Tabak, and M.S. Reddy, Specificity of salivary-bacterial interactions: II. Evidence for a lectin on Streptococcus sanguis with specificity for a NeuAcα2→3Gaiβ1→3GalNac sequence. Biochem. Biophys. Res. Commun. 106:390–396 (1982).
J. Parkkinen, J. Finne, M. Achtman, V. Väisänen and T.K. Korhonen, Escherichia coli strains binding neuraminylα2→3 galactosides. Biochem. Biophys. Res. Commun. III:456–4 61 (1983).
R.J.Gibbons and J.V. Quershi, Selective binding of blood group-reactive salivary mucins by Streptococcus mutans and other oral organisms. Infect. Immun. 22: 665–671 (1978).
K. Landsteiner and R.A. Harte, On group specific A substances. IV. The substance from hog stomach. J.Exp. Med. 72:551–562 (1940).
F.M. Burnet, Mucins and mucoids in relation to influenza virus action. III. Inhibition of virus haemagglutination by glandular mucins. Australian J. Exp. Biol. Med. Sci. 26:311–319 (1948).
A. Gottschalk, Carbohydrate residue of a urine muco- protein inhibiting influenza virus haemagglutination. Nature 170:662–663 (1952).
O. Hammarsten, Uber das Mucin der Submaxillardrüse. I. Darstellung, Zusammensetzung und Eigenschaften des Submaxillarismucins. Hoppe-Seyler’s Z. 12:163–195 (1888).
P. Vaith and G. Uhlenbruck, The Thomsen agglutination phenomenon: a discovery revisited 50 years later. Z. Immun. Forsch. 154:1–14 (1978).
G.W.G. Bird, Anti-T in peanuts. Vox Sang.9: 748–749 (1964).
D.B. Thomas and R.J. Winzler, Structural studies on human erythrocyte glycoproteins alkali-labile oligosaccharides. J. Biol. Chem. 244:5943–5946 (1969).
E. Lisowska, Antigenic Properties of human erythrocyte glycophorins in Molecular Immunology of Complex Carbohydrates. Wu, A.M., Ed. Plenum Press. New York and London (1987).
G.F. Springer, P.R. Desai, M.S. Murthy, H.J. Yang and E.F. Scanlon, Precursors of the blood group MN antigens as human carcinoma-associated antigens. Transfusion 15:223–247 (1979).
R. Schauer, Occurrence of Sialic Acids in Sialic Acids, Chemistry, Metabolism and Function. Springer Verlag, Wien, New York (1982) p. 5–27.
R. Schauer, Chemistry, metabolism and biological functions of sialic acids. Adv. Carbohydrate Chem. Biochem. 40:131–234 (1982).
R. Schauer, Sialic acids and their role as biological masks. Trends Biochem. SCi. 10:357–361 (1985).
D.C. Gowda, V.P. Bhavanandan and E.A. Davidson, Structures of O-linked oligosaccharides present in the proteoglycans secreted by human mammary epithelial cells. J. Biol. Chem. 261:4935–4939 (1986).
J. Haverkamp, R. Schauer and M. Wember, Neuraminic acid derivatives newly discovered in Humåns: N-acetyl-9–0- lactoyl-neuraminic acid, N-9-0-diacetylneuraminic acid and N-acetyl-2,3-dehydro-2-deoxyneuraminic acid. Hoppe- Seyler’s Z. 357:1699–1705 (1976).
G. Tettamanti and W. Pigman, Purification and characterization of bovine and ovine submaxillary mucins. Arch. Biochem. Biophys. 124:41–50 (1968).
W.B. Clarke and R.J. Gibbons, Influence of salivary components and extracellular polysaccharide synthesis from sucrose on the attachment of Streptococcus mutans 6715 to hydroxyapatite surfaces. Infect. Immun. 28:514–523 (1977).
J.M. Creeth, K.R. Bhasker, J.R. Horton, I. Das, M. Lopez- Vidriero and L. Reid, The separation and characterization of bronchial glycoproteins by density gradient methods. Biochem. J. 267:557–569 (1977).
Carlstedt, H. Lindgren, J.K. Sheehan, U. Ulmsten and L. Wingerup, Isolation and characterization of human cervical-mucous glycoproteins. Biochem. J. 211:13–22 (1983).
M. Mantle, D. Mantle and A. Allen, Polymeric structure of pig small-intestinal mucus glycoprotein. Dissociation by proteolysis or by reduction of disulfide bridges. Biochem. J. 195:211–285 (1981).
G. Lamblin, M. Lhermitte, P. Degand, P. Roussel and H. Slayter, Chemical and physical properties of human bronchial mucus glycoproteins. Biochimie 61:23–43 (1979).
C.E. Snyder, C.E. Nadziejko and A. Herp, Isolation of bronchial mucins from cystic fibrosis sputum by use of citraconic anhydride. Carbohydr. Res. 105:81–93 (1982).
N. Fleming, M. Brent, R. Arellano and J.F. Forstner, Purification and immunofluorescent localization of rat submandibular mucin. Biochem. J. 205:225–233 (1982).
K.G. Holden, N.C.F. Yim, L.J. Griggs and J.A. Weisbach, Gel electrophoresis of mucous glycoproteins. I. Effect of gel porosity. Biochemistry 10:3105–3109 (1971).
K.G. Holden, N.C.F. Yim, L.J. Griggs and J.A. Weisbach, Gel electrophoresis of mucous glycoproteins. II. Effect of physical deaggregation and disulfide-bond cleavage. Biochemistry 10:3110–3113 (1971).
N. Payza, M. Robert and A. Herp, The molecular weight of bovine and porcine submaxillary mucins. Int. J. Protein Res.2:109–115 (1970).
S.E. Harding, An analysis of the heterogeneity of mucins. Biochem. J. 219:1061–1064 (1984).
W. Pigman, Submandibular and sublingual glycoproteins. In The Glycoconjugates (M. Horowitz and W. Pigman, Eds.). Vol. 1, 137–152 (1977), Academic Press, Inc. New York.
Nasir-Ud-Din, R.W. Jeanloz, G. Lamblin, P. Roussel, H. Van Halbeek, J.H.G. Mutsaers and J.F.G. Vliegenthart, Structure of sialyloligosaccharides isolated. from bonnet monkey (Macaca radiata) cervical mucus glycoproteins exhibiting blood group activity. J. Biol. Chem. 261: 1992–1997 (1986).
A.M. Wu and W. Pigman, Preparation and characterization of armadillo submandibular glycoproteins. Biochem. J. 161:31–41 (1977).
C.G. Lombart and R.J. Winzler, Isolation and characterization of canine submaxillary mucin. Biochem. J. 128:915–911 (1972).
B.B. Dutta., S. Ghosh, A. Das and C.V.N. Rao, Isolation and characterization of goat submaxillary-mucin. Carbohydr. Res. 101:101–108 (1982).
F. Downs and A. Herp, Chemical studies on a hamster sublingual glycoprotein. Int. J. Peptide Protein Res. 10:229–234 (1977).
F. Downs, M. Harris and A. Herp, The isolation and properties of a glycoprotein from hamster submaxillary gland. Arch. Oral Biol. 21:307–311 (1976).
M.M. Baig, R.J. Winzler and O.M. Rennert, Isolation of mucin from human submaxillary secretions. J. Immunol. 111:1826–1833 (1973).
P.A. Roukema, C.H. Oderkerk and M.S. Salkinoja-Salonen, The murine sublingual and submandibular mucins, their isolation and characterization. Biochim. Biophys. Acta 428:432–440 (1976).
P.A. Denny and P.C. Denny, Purification and biochemical characterization of a mouse submandibular sialomucin. Carbohydr. Res. 57:265–274 (1980).
N. Payza, S. Rizvi and W. Pigman, Studies of action of acids and bases on porcine submaxillary mucin. Arch. Biochem. Biophys. 129:68–14 (1969).
J. Moschera and W. Pigman, The isolation and characterization of rat sublingual mucus-glycoprotein. Carbohydr. Res. 40:53–61 (1975).
L.A. Tabak, L. Mirels, L.D. Monte, A. L. Ridall, M.J. Levine, R.E. Loomis, F. Lindauer, M.S. Reddy and B.J. Baum, Isolation and characterization of a mucin-glycoprotein from rat submandibular glands. Arch. Biochem. Biophys. 242:383–392 (1985).
Y. Hashimoto and W. Pigman, Action of proteolytic enzymes on purified bovine submaxillary mucins. N.Y. Acad. Sci. 106:233–246. (1962).
B. Anderson, N. Seno, P. Sampson, J.G. Riley, P. Hoffman and K. Meyer, Threonine and serine linkage in mucopolysaccharides and glycoproteins. J. Biol. Chem. 239:PC 2716–2717 (1964).
D. M. Carlson, Structures and immunochemical properties of oligosaccharides isolated from pig submaxillary mucins. J. Biol. Chem. 243:616–626 (1968).
F. Downs, A. Herp, J. Moschera and W. Pigman, β-Elimination and reduction reactions and some applications of dimethylsulfoxide on submaxillary glycoproteins. Biochim. Biophys. Acta 328:182–192 (1973).
C-C.W. Chao, J.P. Vergnes and S.I. Brown, O-Glycosidic linkage in glycoprotein isolates from human ocular mucus. Exp. Eye Res. 37:533–541 (1983).
R.D. Marshall, Determination of the 4-N-2-acetamido-2- deoxy-β- D-glucopyranosyl- L-asparagine linkage in glycoproteins. Methods Carbohydr. Chem. 7:212–220 (1976).
R.G. Spiro, Determination of the 5–0-β- D-galactopyrano- sylhydroxy- L-lysine linkage in glycoproteins. Methods Carbohydr. Chem.7:205–211 (1976).
S. Ogata and K.O. Lloyd, Mild alkaline borohydride treatment of glycoproteins — a method for liberating both N- and O-linked carbohydrate chains. Anal. Biochem. 119:351–359 (1982).
J.R. Neeser, G.l.c. of methyloxime and alditol acetate derivatives of neutral sugars, hexosamines, and sialic acids: “one pot” quantitative determination of the carbohydrate constituents of glycoproteins and a study of the selectivity of alkaline borohydride reductions. Carbohydr. Res. 138:189–198 (1985).
E. F. Hounsell, N.J. Pickering, M.S. Stoll, A.M. Lawson and T. Feizi, The effect of mild alkali and alkaline borohydride on the carbohydrate and peptide moieties of fetuin. Biochem. Soc. Trans. 12:607–610 (1984).
H. Debray, G. Strecker and J. Montreuil, Effect of alkalis on N-glycosidic linkages of glycoproteins. Biochem. Soc. Trans. 12:611–612 (1984).
K. Tanaka and W. Pigman, Improvements in hydrogenation procedure for demonstration of 0-threonine glycosidic linkages in bovine submaxillary mucin. J. Biol. Chem. 240:PC1487–1488 (1965).
A. Herp, A.M. Wu and J. Moschera, Current concepts of the structure and nature of mammalian salivary mucous glycoproteins. Mol. Cell. Biochem. 23:27–44 (1979).
F. Downs, C. Peterson, V.L.N. Murty and W. Pigman, Quantitation of the β-elimination reaction as used on glycoproteins. Int. J. Peptide Protein Res. 10:315–322 (1977).
J.F.G. Vliegenthart, L. Dorland and H. Van Halbeek, High resolution 1H-nuclear magnetic resonance spectroscopy as a tool in the structural analysis of carbohydrates related to glycoproteins. Adv. Carbohydr. Chem. Biochem. 41:209–374 (1983).
K. Dill, Natural-abundance, 13C-nuclear magnetic resonance spectral studies of carbohydrates linked to amino acids and proteins. Adv. Carbohydr. Chem. Biochem. 43:1–49 (1985).
J.H.G.M. Mutsaers, H. Van Halbeek, J.F.G. Vliegenthart, A.M. Wu, and E.A. Kabat, Typing of core and backbone domains of mucin-type oligosaccharides from human ovarian- cyst glycoproteins by 500-MHz 1H-NMR spectroscopy. Eur. J. Biochem. 157:139–146 (1986).,
P.A. Denny and P.C. Denny, A mouse submandibular sialomucin containing both N- and O-glycosylic linkages. Carbohydr. Res. 110:305–314 (1982).
A.V. Nieuw Amerongen, C.H. Oderkerk, P.A. Roukema, J.H. Wolf, J.J.W. Lisman and B. Overdijk, Murine submandibular mucin (MSM): a mucin carrying N — and O-glycosylically bound carbohydrate-chains. Carbohydr. Res. 115:C1-C5 (1983).
A.M. Wu, A. Slomiany, A. Herp and B.L. Slomiany, Structural studies on the carbohydrate units of armadillo submandibular glycoprotein. Biochim. Biophys. Acta 575: 297–304 (1979).
A. Slomiany and B.L. Slomiany, Structures of the acidic oligosaccharides isolated from rat sublingual glycoprotein. J. Biol. Chem. 253:7301–7306 (1978).
H.P. Buscher, J.Casals-Stenzel and R. Schauer, Identification of N-glycoloyl-O-acetylneuraminic acids and N-acety1-O-glycoloylneuraminic acids by improved methods for detection of N-acyl and O-acyl groups and by gas-liquid chromatography. Eur. J. Biochem. 50:71–82 (1974).
G. Reuter, R. Pfeil, S. Stoll, R. Schauer, Identification of new sialic acids derived from glycoprotein of bovine submandibular gland. Eur. J. Biochem. 134:139–143 (1983).
J.P. Kamerling, J.F.G. Vliegenthart, C.Versluis and R. Schauer, Identification of O-acetylated N-acylneuraminic acid by mass spectrometry. Carbohydr. Res. 41:1–11 (1975).
B.L.Slomiany, A.Slomiany and A. Herp, Studies on the occurrence of disialosyl groups in glycoproteins of salivary glands. Eur. J. Biochem. 90:255–266 (1978).
S. Ando and R.K. Yu, Isolation and characterization of a novel trisialoganglioside, GTLA from human brain. J. Biol. Chem. 252:6247–6250 (1977).
H.S. Slayter, G. Lamblin, A. Lreut, C. Galabert, N. Houdret, P. Degand and P. Roussel, Complex structure of human bronchial mucus glycoprotein. Eur. J. Biochem. 242:209–218 (1984).
M.W. Leigh, P-W. Cheng, J.L. Carson and T.F. Boat, Developmental changes, in glycoconjugate secretion by ferret tracheas. Am. Rev. Respir. Dis. 234:784–790(1986).
B.L. Slomiany and K. Meyer, Isolation and structural studies of sulfated glycoproteins of hog gastric mucosa. J. Biol. Chem. 247:5062–5070 (1972).
M. Bertolini and W. Pigman, The existence of oligosaccharides in bovine and ovine submaxillary mucins, Carbohydr. Res. 24::53–63. (1970).
T. Tsuji and T. Osawa, Carbohydrate structures of bovine submaxillary mucin. Carbohyd. Res. 151:391–402 (1986).
C.G. Lombart and R.J. Winzler, Isolation and characterization of oligosaccharides from canine submaxillary mucin. Eur. J. Biochem. 49:11–86 (1974).
B. Dutta and C.V.N. Rao, Structures of carbohydrate chains of glycoprotein isolated from goat submaxillary mucin. Biochim. Biophys. Acta 701:12–85 (1982).
M.S. Reddy, M.J. Levine and A. Prakobphol, Oligosaccharide structures of the low-molecular-weight salivary mucin from a normal individual and one with cystic fibrosis. J. Dent. Res. 64:33–36 (1985).
D.H. Van Den Eijnden, W.E.C.M.Schiphorst and E.G. Berger, Specific detection of N-acetylglucosamine containing oligosaccharide chains on ovine submaxillary asialomucin. Biochim. Biophy. Acta 755:32–39 (1983).
H. Van Halbeek, L. Dorland, J. Haverkamp, G.A. Veldink, J.F.G. Vliegenthart, B. Fournet, G. Ricart, J. Montreuil, W. Gathmann and D. Aminoff, Structure determination of oligosaccharides isolated from A+, H+ and A-H- hog- submaxillary gland mucin glycoproteins, by 3 60-MHz 1H-nmr spectroscopy, permethylation analysis and mass spectrometry. Eur. J. Biochem. 118:487–495 (1981).
A. V. Savage, P.L. Koppen, W.E.C.M. Schiphorst, L.A.W. Trippelitz, H. Van Halbeek, J.F.G. Vliegenthart and D.H. Van Den Eijnden, Porcine submaxillary mucin contains α2->3 and α2→6-linked N-acetyl- and N-glycolyl-neuraminic acid. Eur. J. Biochem. 160:123–129 (1986).
N. Payza, L. Martinez and W. Pigman, Immunological and chemical studies on porcine submaxillary mucins. Anim. Blood Groups. Biochem. Genet. 1:195–206 (1970).
R.C. Caldwell and W. Pigman, The carbohydrates of human submaxillary glycoproteins in secretors and non-secretors of blood group substances. Biochim. Biophys. Acta 101:157–165 (1965).
M. Brockhaus, M. Wysocka, J.L. Magnani, Z. Steplewski, H. Koprowski and V. Ginsburg, Normal salivary mucin contains the gastrointestinal cancer-associated antigen detected by monoclonal antibody 19–9 in the serum mucin of patients. Vox Sang. 48:34–38 (1985).
J.M. Wieruszeski, J.C. Michalski, J. Montreuil, G. Strecker, J.P. Katalinic, H. Egge, H. van Halbeek, J.H.G.M. Mutsaers, and J.F.G. Vliegenthart, Structure of the monosialyl oligosaccharides derived from salivary gland mucin glycoproteins of the Chinese swiftlet (genus Collocalla) Characterization of novel types of extended core structure, Galβ(1→3) [GlcNAcβ (1→6)] GalNAcα (1→3) GalNAc(-ol), and of chain termination, [Galα (1→4)] o-1 [Galβ(1→4)]2 GlcNAcβ (1→). J. Biol. Chem. 2 62:6650–6657 (1987).
Gottschalk, The basic structure of glycoproteins and problems of their chemical and physicochemical analysis. N.Y. Acad. Sci. 106:168–176 (1963).
K. Barrett-Bee, G. Bedford and P. Loftus, The use of high resolution carbon-13 NMR in the study of mucus. Adv. Exp. Med. Biol. 144:109–111 (1982).
G.P. Sachdev, J.M. Zodrow and R. Carubelli, Hydrophobic interaction of fluorescent probes with fetuin, ovine submaxillary mucin and canine tracheal mucins. Biochim. Biophys. Acta 580:85–90 (1979).
A.Allen, Mucus — a protective secretion of complexity. Trends Biochem. Sci. 8:169–173 (1983).
G.P. Roberts, The role of disulfide bonds in maintaining the gel structure of bronchial mucus. Arch. Biochem. Biophys. 173:528–537 (1976).
A.0. Jenssen and 0. Smidsrod, Preparation of enzymatically active lysozyme from sputum and its distribution between the sol and gel phases. Eur. J. Respir. Dis. 63:584–590 (1982).
N. Houdret, G. Lamblin, A. Scharfman, D.Humbert and P. Roussel, Activation of bronchial mucin proteolysis by 4- aminophenylmercuric acetate and disulfide reducing agents. Biochim. Biophys. Acta 755:24–29 (1983).
I.P. Williams, R.L. Hall, R.J. Miller and P.S. Richardson, Analyses of human tracheobronchial mucus from healthy subjects. Eur. J. Respir. Dis. 63:510–515 (1982).
P. Roussel, G. Lamblin, N. Houdret, M. Lhermitte and H.S. Slayter, Conformation of human mucus glycoproteins observed by electron microscopy. Biochem. Soc. Trans. 12:617–618 (1984).
A. Gottschalk and H.A. Menzie, Studies on mucoproteins. VIII. On the molecular size and shape of ovine submaxillary gland mucoprotein. Biochim. Biophys. Acta 54:226–235 (1961).
H.D. Hill, Jr., J.A. Reynolds and R.L. Hill, Purification, composition, molecular weight, and subunit structure of ovine submaxillary mucin. J. Biol. Chem. 252:3791–3793 (1977).
H.D. Hill, Jr., M. Schwyzer, H.M. Steiman and R.L. Hill, Ovine submaxillary mucin. Primary structure and peptide substrates of UDP-N-acetylgalactosamine:mucin transferase. J. Biol. Chem. 252:3799–3804 (1977).
J.P. Aubert, G. Biserte, and M.H. Loucheux-Lefebvre, Carbohydrate-peptide linkage in glycoproteins. Arch. Biochem. Biophys. 175:410–418 (1978).
N.J. Maeji, T. Inoue, and R. Chujo, The role of the N- acetyl group in determining the conformation of 2 acetamido-2-deoxy-D-galactopyranosyl-threonine-containing peptides. Carbohydr. Res. 162:C4-C8 (1987).
V.P. Bhavanandan and J.D. Hegarty, Identification of the mucin core protein by cell-free translation of messenger RNA from bovine submaxillary glands. J. Biol. Chem. 262:5913–5917 (1987).
M.C. Rose, W.A. Voter, H. Sage, ’ C.F. Brown and B. Kaufman, Effects of deglycosylation of the architecture of ovine submaxillary mucin glycoprotein. J. Biol. Chem. 259;3167–3172 (1984).
N. Jentoft, R.S. Shogren and T. A. Gerken, The conformation of mucins and O-glycosylated membrane proteins. Federation Proc. 45:2150 (1987).
R.L. Shogren, N. Jentoft, T.A. Gerken, A.M. Jamieson, and J. Blackwell, Light-scattering studies of fractionated ovine submaxillary mucin. Carbohydr. Res. 160:311–328 (1987).
R. Shogren, A.M. Jamieson and J. Blackwell, Solution properties of porcine submaxillary mucin. Biopolymers 22:1657–1675 (1983).
W.T.J. Morgan and W.M. Watkins, The inhibition of the haemmagglutinins in plant seeds by human blood group substances and simple sugars. Brit. J. Exp. Pathol. 34:94–103 (1953).
H. Lis and N. Sharon, Lectins as molecules and as tools. Ann. Rev. Biochem. 55:35–67 (1986).
G. Ashwell and J. Harford, Carbohydrate-specific receptors of the liver. Ann. Rev. Biochem. 51:531–554 (1982).
I.E. Liener, N. Sharon, and I.J. Goldstein, The Lectins. Properties, Functions, and Applications in Biology and Medicine. Academic Press, Orlando, FL, (1986).
I.J. Goldstein and I.E. Etzler, Chemical Toxonomy, Molecular Biology and Function of Plant Lectins. Alan R. Liss. New York, (1983).
G.G. Sahagian, The mannose 6-phosphate receptor: function, biosynthesis and translocation. Biol. Cell 51:207–214 (1984).
M. A. Lehrman and R.L. Hill, The binding of fucose containing glycoproteins by hepatic lectins. Purification of a fucose-binding lectin from rat liver. J. Biol. Chem. 261:7419–7425 (1986).
E.F. Neufeld and G. Ashwell, Carbohydrate recognition systems for receptor-mediated pinocytosis. In The Biochemistry of Glycoproteins and Proteoglycans (W.J. Lennarz, Ed.). Plenum Press, New York, pp 241–266 (1980).
A.M. Wu, Differential binding characteristics and applications of DGalβ1→3DGalNac specific lectins, Mol. Cell. Biochem. 61:131–141 (1984).
A.M. Wu and A. Herp, A table of lectin carbohydrate specificities. In Lectins (T.C. Bøg-Hansen and J. Breborowicz, Eds.). W. de Gruyter & Co., New York, Vol. IV., pp 629–636, (1985).
P.J.A. Holt, J.H. Anglin and R.E. Nordquist, Localization of specific carbohydrate configurations in human skin using fluorescein-labeled lectins. Br. J. Dermatol. 100:237–245 (1979).
G.L. Nicolson and S.J. Singer, The distribution and asymmetry of mammalian cell surface saccharides utilizing ferritin-conjugated plant agglutinins as specific saccharide stains. J. Cell Biol. 60:236–248 (1974).
K. Burridge, Direct identification of specific glycoproteins and antigens in sodium dodecyl sulfate gels, Methods Enzymol. 50:54–64 (1978).
E.V. Crean and E.F. Rosomando, Developmental changes in membrane-bound enzymes of Dictyostelium discoideum detected by concanavalin A-Sepharose affinity chromatography. Biochem. Biophys. Res. Commun. 75:488–495 (1977).
I.J. Goldstein, C.E. Hollerman and E.E. Smith, Protein- carbohydrate interaction. II. Inhibition studies on the interaction of concanavalin A with polysaccharides. Biochemistry 4:876–883 (1965).
Y.Ch. Sekharudu, M. Biswas and V.S.R. Rao, Complex Carbohydrates: 2. The modes of binding of complex carbohydrates to concanavalin A-a computer modelling approach. Int. J. Biol. Marcomol:8:9–19 (1986).
A.M. Wu, E.A. Rabat, F.G. Gruezo, and H.J. Allen, Immunochemical studies on the combining site of the D- galactopyranose and 2-acetamido-2-deoxy-D-galactopyranose specific lectin isolated from Bauhinia purpurea alba seeds. Arch. Biochem. Biophys 204:622–639 (1980).
D. A. Baker, S. Sugii, E.A. Rabat, R.M. Ratcliffe, P. Hermentin and R.U. Lemieux, Immunochemical studies on the combining sites of Forssman hapten reactive hemagglutinins from Dolichos biflorus, Helix pomatia, and Wistaria floribunda. Biochemistry 22:2741–2750 (1983).
E.A. Rabat, A. Bendich, A. E. Bezer, and S.M. Beiser, Immunochemical studies on blood groups. IV. Preparation of blood group A substances from human sources and a comparison of their chemical and immunochemical properties with those of the blood group A substance from hog stomach. J. Exp. Med. 85:685–699 (1947).
E. A. Rabat, A. Bendich, A. E. Bezer and V. Knaub, Immunochemical studies on blood groups. VI. The cross- reaction between type XIV antipneumococcal horse serum and purified blood group A, B, and 0 substances from hog and human sources, J. Exp. Med. 87:295–300 (1948).
H.H. Baer, E.A. Kabat, and V. Knaub, Immunochemical studies on blood groups. X. The preparation of blood group A and B substances and an inactive substance from individual horse stomachs and of blood group B substance from human saliva. J. Exp. Med. 91, 105–114 (1950).
W.M. Watkins, Blood group specific substances. In Glycoproteins, 2nd ed. (A. Gottschalk, ed.). Part B. pp 830–891.(1972) Elsevier Publ., New York.
A.M. Wu, E.A. Kabat, F.G. Gruezo and R.D. Poretz, Immunochemical studies on the reactivities and combining sites of the D-galactopyranose- and 2-acetamido-2-deoxy-D- galactopyranose-specific lectin purified from Sophora japónica seeds. Arch. Biochem. Biophys. 209:191–203 (1981).
M.S. Sarkar, A.M. Wu and E. A. Kabat, Immunochemical studies on the carbohydrate specificity of Madura pomífera lectin, Arch Biochem. Biophys. 209:204–218 (1981).
Y. Takai, Y. Noda, S. Sumitono, S. Sagara and M. Mori, Different bindings to lectin in human submandibular gland after enzymatic digestion. Acta Histochem. 78:111–121 (1986).
M.E.A. Pereira, E.A. Kabat, R. Lotan, and N. Sharon, Immunochemical studies on the specificity of the peanut (Arachis hypogaea) agglutinin, Carbohyd. Res. 51:107–118 (1976).
T. Irimura and T. Kawaguchi, T. Terao and T. Osawa, Carbohydrate-binding specificities of the so-called galactose-specific phytohemagglutinins. Carbohyd. Res. 39: 317–327 (1975).
T Osawa, T. Irimura and T. Kawaguchi, Bauhinia purpurea agglutinin. Methods Enzymol. 50:361–312 (1978).
A.C. Roche, R. Schauer and M. Monsigny, Protein-sugar interactions. Purification by affinity chromatography of limulin, and N-acyl-neuraminidyl-binding protein, FEBS Let. 57:245–249 (1975).
K. Furukawa, J.E. Minor, J.D. Hegarty and V.P. Bhavanandan, Interaction of sialoglycoproteins with wheat germ agglutinin-Sepharose of varying ratio of lectin to Sepharose. Use for the purification of mucin glycoproteins from membrane extracts. J. Biol. Chem. 261:1155-7761 (1986).
V.P. Bhavanandan and A.W. Katlic, The interaction of wheat germ agglutinin with sialoglycoproteins. The role of sialic acid. J. Biol. Chem. 254:4000–4008 (1979).
T. Menghi, A.M. Bondi, D. Accili, L. Fumagalli and G. Materazzi, Characterizationin situ of the complex carbohydrates in rabbit oviduct using digestion with glycosidases followed by lectin binding, J. Anat. 140:613–625 (1985).
T. Faraggiana, D. Villari, J. Jagirdar and J. Patil, Expression of sialic acid on the alveolar surface of adult and fetal human lungs. J. Histochem. Cytochem. 34:811–816 (1986).
S.A. Laden, B.A. Schulte and S.S. Spicer, Histochemical evaluation of secretory glycoproteins in human salivary glands with lectin-horseradish peroxidase conjugates. J. Histochem. Cytochem. 32:965–972 (1984).
B.A. Schulte and S.S. Spicer, Light microscopic detection of sugar residues in glycoconjugates of salivary glands and the pancreas with lectin-horseradish peroxidase conjugates. I. Mouse. Histochem. J. 15:1217–1238
B.A. Schulte and S.S. Spicer, Light microscopic detection of sugar residues in glycoconjugates of salivary glands and the pancreas with horseradish-peroxidase conjugates. II. Rat. Histochem. J. 16:3–20 (1984).
P.A. Murray, M.J. Levine, L. A. Tabak and M.S. Reddy, Purification of a sialic acid binding lectin from Streptococcus mitis. Soc. Complex Carbohydr. Annual Meeting, 44 (1983).
O. Sobeslavsky, B. Prescott and R.M. Chanock, Adsorption of Mycoplasma pneumoniae to neuraminic acid receptors of various cells and possible role in virulence. J. Bacteriol. 96:695–705 (1968).
B.C. McBride and M.T. Gisslow, Role of sialic acid in saliva-induced aggregation of Streptococcus sanguis. Infect. Immun. 18:35–40 (1977).
M.J. Levine, M.C. Herzberg, M.S. Levine, S.A. Ellison, M.W. Stinson, H.C. Li and T. van Dyke, Specificity of salivary-bacterial interactions: role of terminal sialic acid residues in the interaction of salivary glycoproteins with Streptococcus sanguis and Streptococcus mutans. Infect. Immun. 19:101–115 (1978).
T.Ericson and J. Rundegen, Characterization of a salivary agglutinin with a serotype c strain of Streptococcus mutans. Eur. J. Biochem. 133:255–261 (1983).
C.W.I. Douglas and R.R.B. Russell, The adsorption of human salivary components to strains of the bacterium Streptococcus mutans. Arch. Oral Biol. 25:751–757.(1984)
J.P. Babu, S.N. Abraham, M.K. Dabbous and E.H. Beachey, Interaction of a 60-kilodalt on D-mannose-cont aining salivary glycoprotein with type i fimbriae of Escherichia coli. Infect. Immun. 54:104–108 (1986).
T. Feizi, Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature 314:53–57 (1985).
C.E. Snyder, C.E. Nadziejko and A. Herp, Human bronchial explants in long-term culture: establishing a baseline for secretion. In Vitro 20:95–102 (1984).
D.A. Sens, D.S. Hintz, M.T. Rudisiii, M.A. Sens and S.S. Spicer, Methods in laboratory investigation. Explant culture of human submandibular gland epithelial cells: evidence of ductal origin. Lab. Invest. 52:557–567 (1985).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1988 Plenum Press, New York
About this chapter
Cite this chapter
Herp, A., Borelli, C., Wu, A.M. (1988). Biochemistry and Lectin Binding Properties of Mammalian Salivary Mucous Glycoproteins. In: Wu, A.M., Adams, L.G. (eds) The Molecular Immunology of Complex Carbohydrates. Advances in Experimental Medicine and Biology, vol 228. Springer, Boston, MA. https://doi.org/10.1007/978-1-4613-1663-3_15
Download citation
DOI: https://doi.org/10.1007/978-1-4613-1663-3_15
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4612-8923-4
Online ISBN: 978-1-4613-1663-3
eBook Packages: Springer Book Archive