Abstract
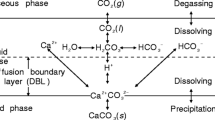
Carbonate speleothems and precipitating fluids from Carlsbad Caverns, New Mexico, have been analyzed for their major and minor element and stable isotopic compositions in order to evaluate processes controlling the chemical evolution of cave water and factors determining the mineralogy and composition of cave carbonates. Chemistry and isotopic composition of fluids are determined by rates of CO2 degassing, evaporation, and carbonate precipitation. Evaporation and calcium carbonate precipitation cause changes in the Mg/Ca ratio of fluids which, coupled with changes in CO3 = content, control the minor element chemistry and mineralogy of the precipitating phase.
A broad range of carbonate minerals precipitate from seepage cave fluids, with calcites containing 1.5 to 12.0 mole % MgCO3; calcite Mg contents exhibit a nonlinear dependence on fluid Mg/Ca ratio, and a linear dependence on fluid CO3 = content, indicating dual control by both cation and anion con-centrations. Calcite-aragonite polymorphism appears to be largely controlled by elevated fluid Mg/Ca ratios. Combined water and carbonate chemical and stable isotopic data suggest that the most Mg- enriched calcites (10 to 12 mole % MgCO3) either coprecipitate with aragonite or precipitate from waters with higher CO2= concentrations than those precipitating aragonite. The range of calcite compositions associated with aragonite suggest that it coprecipitates with Mg-depleted calcite at low fluid CO3= concentrations, while Mg-enriched calcites form with aragonite at high CO3= concentrations. Hydromagnesite and huntite precipitate under conditions of extreme evaporation at elevated fluid Mg concentrations and Mg/Ca ratios. Primary dolomite precipitates from waters of moderate Mg/Ca ratio, probably from fluids undersaturated with respect to calcite and aragonite.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Berner, R.A., 1975, The role of magnesium in the crystal growth of calcite and aragonite from sea water: Geochim. Cosmochim. Acta, v. 39, p. 489–504.
Bischoff, J.L., 1968, Kinetics of calcite nucleation: magnesium ion inhibition and ionic strength catalysis: Jour. Geophys. Res., v. 73, p. 3315–3322.
Cabrol, P., and Coudray, J., 1982, Climatic fluctuations influence the genesis and diagenesis of carbonate speleothems in southwestern France: Nat. Spel. Soc. Bull., v. 44, p. 112–117.
Cave Research Foundation, 1979, Map of Carlsbad Caverns National Park: Cave Research Foundation, Washington, DC, 1 p.
Craig, H., 1957, Isotopic standards for carbon and oxygen and correction factors for mass-spectro- metric analysis of carbon dioxide: Geochim. Cosmochim. Acta, v. 12, p. 133–149.
Craig, H., Gordon, L.I., and Horibe, Y., 1963, Isotopic exchange effects in the evaporation of water: Jour. Geophys. Res., v. 68, p. 5079–5087.
Deines, P., Langmuir, D., and Harmon, R.S., 1974, Stable carbon isotope ratios and the existence of a gas phase in the evolution of carbonate ground waters: Geochim. Cosmochim. Acta, v. 38, p. 1147–1164.
Epstein, S., and Mayeda, T., 1953, Variation of O18 content of waters from natural sources: Geochim. Cosmochim. Acta, v. 4, p. 213–224.
Fantidis, J., and Ehhalt, D.H., 1970, Variations of the carbon and oxygen isotopic composition in stalagmites and stalactites: evidence of nonequi- librium isotopic fractionation: Earth Planet. Sci. Lett., v. 10, p. 136–144.
Fornaca-Rinaldi, G., Panichi, C., and Tongiori, E., 1968, Some causes of the variation of the isotopic composition of carbon and oxygen in cave concretions: Earth Planet. Sci. Lett., v. 4, p. 321–324.
Given, R.K., and Wilkinson, B.H., 1985, Kinetic control of morphology, composition, and mineralogy of abiotic sedimentary carbonates: Jour. Sed. Petrol., v. 55, p. 109–119.
Gonzalez, L.A., and Lohmann, K.C, 1985, Carbon and oxygen isotopic composition of Holocene reefal carbonates: Geology, v. 13, p. 811–814.
Harmon, R.S., 1979, An isotopic study of groundwater seepage in the central Kentucky karst: Water Resources Res., v. 15, p. 476–480.
Harmon, R.S., Atkinson, T.C., and Atkinson, J.L., 1983, The mineralogy of Castleguard Cave, Columbia Icefields, Alberta, Canada: Arctic Alpine Res., v. 15, p. 503–516.
Holland, H.D., Kirsipu, T.V., Huebner, J.S., and Oxburgh, U.M., 1964, On some aspects of the chemical evolution of cave waters: Jour. Geol., v. 72, p. 36–67.
Inoue, H., and Sugimura, Y., 1985, Carbon isotopic fractionation during the C02 exchange process between air and seawater under equilibrium and kinetic conditions: Geochim. Cosmochim. Acta, v. 49, p. 2453–2460.
Lloyd, R.M., 1966, Oxygen isotope enrichment of seawater by evaporation: Geochim. Cosmochim. Acta, v. 30, p. 801–814.
Moore, G.W., 1956, Aragonite speleothems as indicators of paleotemperature: Amer. Jour. Sci., v. 254, p. 746–753.
Mucci, A., and Morse, J.W., 1983, The incorporation of Mg+ 4- and Sr+ + into calcite overgrowths: influences of growth rate and solution composition: Geochim. Cosmochim. Acta, v. 47, p. 217–233.
Murray, J.W., 1954, The deposition of calcite and aragonite in caves: Jour. Geol., v. 62, p. 481–492.
Murray, J.W., 1975, Additional data on the mineralogy of the New River Cave: Nat. Spel. Soc. Bull., v. 37, p. 79–82.
Plummer, L.N., and Mackenzie, F.T., 1974, Predicting mineral solubility from rate data: application to the dissolution of magnesian calcites: Amer. Jour. Sci., v. 274, p. 61–83.
Rogers, B.W., and Williams, K.M., 1982, Mineralogy of Lilburn Cave, Kings Canyon National Park, California: Nat. Spel. So, c. Bull., v. 44, p. 23–31.
Rubinson, M., and Clayton, R.N., 1969, Carbon-13 fractionation between aragonite and calcite: Geo- chim. Cosmochim. Acta: v. 33, p. 997–1002.
Sears, S.O., 1976, Inorganic and isotopic geochemistry of the unsaturated zone in a carbonate terrain: Ph.D. thesis, Pennsylvania State University, 236 p.
Siegel, F.R., 1965, Aspects of calcium carbonate deposition in Great Onyx Cave, Kentucky: Sedi- mentology, v. 4, p. 285–299.
Tarutani, T., Clayton, R.N., and Mayeda, T., 1969, The effect of polymorphism and magnesium substitution on oxygen isotope fractionation between calcium carbonate and water: Geochim. Cosmochim. Acta, v. 33, p. 987–996.
Thorstenson, D.C., and Plummer, N.L., 1977, Equilibrium criteria for two-component solids reacting with fixed composition in an aqueous phase-ex- ample: the magnesian calcites: Amer. Jour. Sci., v. 277, p. 1203–1223.
Thrailkill, J., 1965, Studies in the excavation of limestone caves and the deposition of speleothems: Part I. Chemical and hydrologic factors in the excavation of limestone caves. Part II. Water chemistry and carbonate speleothem relationships in Carlsbad Caverns, New Mexico: Ph.D. thesis, Princeton University, 193 p.
Thrailkill, J., 1968, Dolomite cave deposits from Carlsbad Caverns: Jour. Sed. Pet., v. 38, p. 141–145.
Thrailkill, J., 1971, Carbonate deposition in Carlsbad Caverns: Jour. Geol., v. 79, p. 683–695.
Tietz, G.F., 1981, Hollow calcite crystals on surfaces of small pools in the Liethiihle/Sauerland, West Germany, in Beck, B.F., ed., Proceedings of the Eighth International Congress of Speleology: v. 1, p. 362–363.
Walter, L.M., 1985, Relative reactivity of skeletal carbonates during dissolution: implications for diagenesis, in Schneidermann, N., and Harris, P.M., eds., Carbonate cements: Soc. Econ. Paleontologists and Mineralogists, Spec. Publ. 36, p. 3–16.
Wilson, W.L., and Ash, D.W., 1985, Stratigraphy of the New Mexico and Guadalupe Rooms in Carlsbad Caverns, New Mexico, in Lindsley, K.B., ed., Annual report: Cave Res. Found. 1984, p. 13–15.
Yonge, C.J., Ford, D.C., Gray, J., and Schwarcz, H.P., 1985, Stable isotope studies of cave seepage water: Chem. Geol., v. 58, p. 97–105.
Zeller, E.J., and Wray, J.L., 1956, Factors influencing precipitation of calcium carbonate: Amer. Assoc. Petroleum Geologists Bull., v. 40, p. 140–152.
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1988 Springer-Verlag New York Inc.
About this chapter
Cite this chapter
Gonzalez, L.A., Lohmann, K.C. (1988). Controls on Mineralogy and Composition of Spelean Carbonates: Carlsbad Caverns, New Mexico. In: James, N.P., Choquette, P.W. (eds) Paleokarst. Springer, New York, NY. https://doi.org/10.1007/978-1-4612-3748-8_4
Download citation
DOI: https://doi.org/10.1007/978-1-4612-3748-8_4
Publisher Name: Springer, New York, NY
Print ISBN: 978-0-387-96563-5
Online ISBN: 978-1-4612-3748-8
eBook Packages: Springer Book Archive