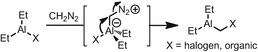Abstract
Diazo compounds continue both to challenge and to fascinate practitioners of chemical synthesis. The most strategically powerful and unique type of reactivity observed with these reagents is a formal insertion of the donor-acceptor carbon into C–C or C–H bonds alpha to carbonyl groups. Although the reaction does not involve discrete carbon–metal bonds, it can be catalyzed by metal-based Lewis acids. This chapter investigates both classical and modern developments in diazoalkyl carbon insertion with a special emphasis on nonstabilized nucleophiles.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Notes
- 1.
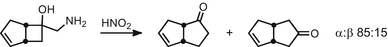
Interestingly, Tiffeneau–Demjanov rearrangement provides mostly the α-ketone product in an 85:15 ratio of regioisomers (determined by IR spectroscopy); see [83])
- 2.
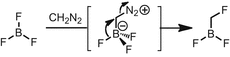
See [90].
- 3.
See [102].
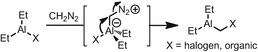
- 4.
Readily prepared by treating trimethylaluminum with two equivalents of BHT; see [99].
- 5.
The cis/trans configuration of 2-methyl-5-tert-butylcycloheptanone was established following equilibration in methanolic NaOCH3.
- 6.
The less electron-poor, more hindered Lewis acids (Sc(acac)3 and Sc(tmhd)3) were substituted for Sc(OTf)3 in reactions with more Lewis basic diazoalkanes in order to maximize the yield of product (tmhd = 2,2,6,6-tetramethyl-3,5-heptanedianato, or tert-butyl(acac)). See [99] and citations therein for more details on the Lewis-acid mediated destruction of diazoalkanes.
- 7.
p-(Methoxy)phenyldiazomethane is a potent Lewis base and has been reported to decompose at temperatures as low as –80° C; see [125].
- 8.
- 9.
Iodobenzene bis(trifluoroacetate) can substitute for lead(IV) acetate with little or no impact on the yield of diazoalkane, but the nonpolar extract becomes contaminated by residual iodobenzene.
References
Curtius T (1883) Ber Dtsch Chem Ges 16:2230
Pechmann HV (1891) Ber Dtsch Chem Ges 27:1888
Gutsche CD (1954) Org Reactions 8:364
Gutsche CD, Redmore D (1968) In: Hart H, Karabatsos GJ (eds) Carbocyclic ring expansion reactions. Academic, New York, pp 81–98
Holmquist CR, Roskamp EJ (1989) J Org Chem 54:3258
Holmquist CR, Roskamp EJ (1992) Tetrahedron Lett 33:1131
Bug T, Hartnagel M, Schlierf C, Mayr H (2003) Chem Eur J 9:4068
Mayr H, Bug T, Gotta MF, Hering N, Irrgang B, Janker B, Kempf B, Loos R, Ofial AR, Remennikov G, Schimmel H (2001) J Am Chem Soc 123:9500
Regitz M, Maas G (1986) Diazo compounds–properties and synthesis. Academic, Orlando
Doyle MP, McKervey MA, Ye T (1998) Modern catalytic methods for organic synthesis with diazo compounds. Wiley, New York
Ye T, McKervey MA (1994) Chem Rev 94:1091
De Boer TJ, Backer HJ (1963) Organic Syntheses Collective, vol 4. Wiley, New York, p 250
Hudlicky M (1980) J Org Chem 45:5377
Clark J, Shah A, Peterson J, Patelis L, Kersten R, Heemskerk A, Grogan M, Camden S (2002) Thermochim Acta 386:65
Clark J, Shah A, Peterson J, Patelis L, Kersten R, Heemskerk A (2002) Thermochim Acta 386:73
Proctor LD, Warr AJ (2002) Org Process Res Dev 6:884
Furrow ME, Myers AG (2004) J Am Chem Soc 126:12222
Kühnel E, Laffan DDP, Lloyd-Jones GC, Martínez Del Campo T, Shepperson IR, Slaughter JL (2007) Angew Chem Int Ed 46:7075
Roberts JD, Watanabe W (1950) J Am Chem Soc 72:4869
Roberts JD, Watanabe W, McMahon RE (1951) J Am Chem Soc 73:760
Roberts JD, Watanabe W, McMahon RE (1951) J Am Chem Soc 73:2521
Roberts JD, Regan CM (1952) J Am Chem Soc 74:3695
Müller E, Bauer M, Rundel W (1959) Z Naturforsch 14b:209
Müller E, Rundel W (1958) Angew Chem 70:105
Müller E, Meischkeil R, Bauer M (1964) Annalen 677:55
Müller E, Rundel W, Huber-Emden H (1957) Angew Chem 69:614
Müller E, Huber-Emden H (1961) Annalen 649:70
Müller E, Huber-Emden H, Rundel W (1959) Annalen 623:34
Neeman M, Caserio MC, Roberts JD, Johnson WS (1959) Tetrahedron 6:36
Neeman M, Caserio MC, Roberts JD, Johnson WS (1958) J Am Chem Soc 80:2584
Pechmann HV (1895) Ber Dtsch Chem Ges 28:855
Smith LI (1938) Chem Rev 23:193
Huisgen R, Grashey R, Sauer J (1964) In: Patai S (ed) The chemistry of alkenes. Interscience, London, p 739
Huisgen R (1968) J Org Chem 33:2291
Atherton JH, Fields R (1968) J Chem Soc C 1507
Barlow MG, Hazseldine RN, Morton WD (1969) Chem Commun 931
Manecke G, Schenck HG (1968) Tetrahedron Lett 9:2061
Tabushi I, Takagi K, Okano M, Oda R (1967) Tetrahedron 23:2621
D’Yakonov IA, Repinskaya IB, Golodnikov GV (1966) Zh Org Khim 2:2256
Kadaba PK (1966) Tetrahedron 22:2453
Ledwith A, Parry D (1966) J Chem Soc B 1408
Doyle MP (1986) Chem Rev 86:919
Doyle MP, Forbes DC (1998) Chem Rev 98:911
Aggarwal VK, Ferrara M, O’Brien C, Thompson A, Jones R, Fieldhouse R (2001) J Chem Soc Perk T 1 1635
Aggarwal VK, Alonso E, Fang G, Ferrara M, Hynd G, Porcelloni M (2001) Angew Chem Int Ed 40:1433
Aggarwal VK, Alonso E, Bae I, Hynd G, Lydon KM, Palmer MJ, Patel M, Porcelloni M, Richardson J, Stenson RA, Studley JR, Vasse J, Winn CL (2003) J Am Chem Soc 125:10926
Acevedo O, Ross B, Andrews RS, Springer R, Cook PD (1995) Apparatus and processes for the large scale generation and transfer of diazomethane (Isis Pharmaceuticals). US Patent 549243
Archibald TG, Huang DS, Pratton MH, Barnard JC (1998) Large scale batch process for diazomethane (Aerojet General Corporation). US Patent 5,817,778
Archibald TG, Barnard JC, Harlan RF (1998) Continuous process for diazomethane from an N-methyl-N-nitrosoamine and from methylurea through N-methyl-N-nitrosourea (Aerojet General Corporation). US Patent 5,854,405
Maas G (2009) Angew Chem Int Ed 48:8186
Myers EL, Raines RT (2009) Angew Chem Int Ed 48:2359
Holton TL, Shechter H (1995) J Org Chem 60:4725
McGuiness M, Shechter H (2002) Tetrahedron Lett 43:8425
Javed MI, Brewer M (2007) Org Lett 9:1789
Wender PA, Handy ST, Wright DL (1997) Chem Ind 765
Buchner E, Curtius T (1885) Ber Dtsch Chem Ges 18:2377
Pechmann HV, Frobenius L (1895) Ber Dtsch Chem Ges 28:170
Meyer H (1905) Monatsh Chem 26:1295
Schlotterbeck F (1907) Ber Dtsch Chem Ges 40:479
Schlotterbeck F (1909) Ber Dtsch Chem Ges 42:2559
Eistert B (1948) Newer methods of preparative organic chemistry. Interscience, New York, p 521, English edition
Meerwein H, Burneleit W (1928) Ber Dtsch Chem Ges 61:1840
Meerwein H (1933) Verfahren zur Umsetzung Organischer Verbindungen mit Diazomethan. German Patent 579,309
Smith PAS, Baer DR (1960) Org Reactions 11:157
Woodward RB, Gosteli J, Ernest I, Friary RJ, Nestler G, Raman H, Sitrin R, Suter C, Whitesell JK (1973) J Am Chem Soc 95:6853
Mosettig E, Burger A (1930) J Am Chem Soc 52:3456
Heller G (1919) Ber Dtsch Chem Ges 52:741
Heller G (1926) Ber Dtsch Chem Ges 59:704
Giraitis AP, Bullock JL (1937) J Am Chem Soc 59:951
Kohler EP, Tishler M, Potter H, Thompson HT (1939) J Am Chem Soc 61:1057
Adamson DW, Kenner J (1939) J Chem Soc 181
Gutsche CD (1949) J Am Chem Soc 71:3513
Gutsche CD, Strohmayer HF, Chang JM (1958) J Org Chem 23:1
Hammett LP (1937) J Am Chem Soc 59:96
Goodman RM, Kishi Y (1998) J Am Chem Soc 120:9392
Nakamura K, Osamura Y (1993) J Am Chem Soc 115:9112
Berson JA, Suzuki S (1959) J Am Chem Soc 81:4088
Gutsche CD, Johnson HE (1955) J Am Chem Soc 77:109
Gutsche CD, Johnson HE (1955) Org Synth 35:91
Gutsche CD, Jason EF (1956) J Am Chem Soc 78:1184
Greene AE, Depres JP (1979) J Am Chem Soc 101:4003
Jaz J, Davreux JP (1965) Bull Chim Soc Belg 74:370
Roberts JD, Gorham WF (1952) J Am Chem Soc 74:2278
Reeder LM, Hegedus LS (1999) J Org Chem 64:3306
Black TH (1983) Aldrichim Acta 16:3
Sammakia T (1995) In: Paquette LA (ed) Encyclopedia of reagents for organic synthesis. Wiley, Chichester, UK, p 1512
Bamford WR, Stevens TS (1952) J Chem Soc 4735
Müller E, Bauer M, Rundel W (1960) Tetrahedron Lett 1:30
House HO, Grubbs EJ, Gannon WF (1960) J Am Chem Soc 82:4099
Goubeau J, Rohwedder KH (1957) Liebigs Ann Chem 604:168
Tai WT, Warnhoff EW (1964) Can J Chem 42:1333
Hashimoto N, Aoyama T, Shioiri T (1980) Tetrahedron Lett 21:4619
Brook AG (1974) Acc Chem Res 7:77
Moser WH (2001) Tetrahedron 57:2065
Brook AG, Limburg WW, MacRae DM, Fieldhouse SA (1967) J Am Chem Soc 89:704
Corey EJ, Rücker C (1984) Tetrahedron Lett 25:4345
Shioiri T, Aoyama T, Mori S (1990) Org Synth 68:1
Kemsley JN (2011) Chem Eng News 89:15
Maruoka K, Concepcion AB, Yamamoto H (1994) Synthesis 1283
Maruoka K, Concepcion AB, Yamamoto H (1994) J Org Chem 59:4725
Müller E, Bauer M (1962) Liebigs Ann Chem 654:92
Hoberg H (1966) Angew Chem Int Ed 5:513
Youn J-H, Lee J, Cha JK (2001) Org Lett 3:2935
Johnson WS, Neeman M, Birkeland SP, Fedoruk NA (1962) J Am Chem Soc 84:989
Moebius DC, Kingsbury JS (2009) J Am Chem Soc 131:878
Moebius DC (2011) Ph.D. Dissertation, Boston College, Chestnut Hill
Evans DA, Fandrick KR, Song H-J, Scheidt KA, Xu R (2007) J Am Chem Soc 129:10029
Wu J (2005) Ph.D. Dissertation, Harvard University, Cambridge
Shankar BKR, Shechter H (1982) Tetrahedron Lett 23:2277
Rendina VL, Kaplan HZ, Kingsbury JS (2012) Synthesis 686
Dabrowski JA, Moebius DC, Wommack AJ, Kornahrens AF, Kingsbury JS (2010) Org Lett 12:3598
Seyferth D, Dow AW, Menzel H, Flood TC (1968) J Am Chem Soc 90:1080
Hashimoto N, Aoyama T, Shioiri T (1982) Chem Pharm Bull 30:119
Shioiri T, Aoyama T, Mori S (1990) Org Synth 68:1
Fleming I, Sanderson PEJ (1987) Tetrahedron Lett 28:4229
Wommack AJ, Moebius DC, Travis AL, Kingsbury JS (2009) Org Lett 11:3202
Wommack AJ, Kingsbury JS (2013) J Org Chem 78:10573–10587
Rendina VL, Moebius DC, Kingsbury JS (2011) Org Lett 13:2004
Arndt F (1935) Org Synth 15:3
Harman WW, Phillips R (1933) Org Synth 13:84
de Boer TJ, Backer HJ (1956) Org Synth 36:16
Lieser T, Beck G (1950) Berichte 83:137
Hart H, Brewbaker JL (1969) J Am Chem Soc 91:706
Wiseman JR, Chan H (1970) J Am Chem Soc 92:4749
Miyashita M, Yoshikoshi A (1974) J Am Chem Soc 96:1917
Thomas RC, Fritzen EL (1988) J Antibiot 41:1445
Fulton J, Aggarwal VK, de Vincente J (2005) Eur J Org Chem 1479
Aggarwal VK, Ford JG, Thompson A, Jones RVH, Standen MCH (1996) J Am Chem Soc 118:7004
Aggarwal VK (1998) Synlett 329
Aggarwal VK, Vasse J (2003) Org Lett 5:3987
Adams LA, Aggarwal VK, Bonnert RV, Bressel B, Cox RJ, Shepherd J, de Vincente J, Walter M, Whittingham WG, Winn CL (2003) J Org Chem 68:9433
Aggarwal VK, de Vincente J, Bonnert RV (2001) Org Lett 3:2785
Angle SR, Neitzel ML (2000) J Org Chem 65:6458
Aggarwal VK, de Vincente J, Pelotier B, Holmes IP, Bonnert RV (2000) Tetrahedron Lett 41:10327
Trost BM, Higuchi RI (1996) J Am Chem Soc 118:10094
Zhang J, Chan P, Che C (2003) Tetrahedron Lett 44:8733
Hu W, Timmons DJ, Doyle MP (2002) Org Lett 4:901
Pross A, Sternhill S (1970) Aust J Chem 23:989
Overberger CG, Anselme J (1964) J Org Chem 29:1188
Abelt CJ, Pleier JM (1989) J Am Chem Soc 111:1795
Smith LI, Howard KL (1944) Org Synth 24:53
Kotali A (2002) Curr Org Chem 6:965
Gale D, Middleton WJ, Krespan C (1966) J Am Chem Soc 88:3617
Ciganek E (1970) J Org Chem 35:862
Bernard RE (1967) Ph.D. Dissertation, The Ohio State University, Columbus
McBee ET, Sienkowski KJ (1973) J Org Chem 38:1340
Ciganek E (1965) J Am Chem Soc 87:652
Creary X (1986) Org Synth 64:207
McGuiness M, Shechter H (1990) Tetrahedron Lett 31:4987
Barton DHR, O’Brien RE, Sternhell S (1962) J Chem Soc 470
Adamson J, Bywood R, Eastlick DT, Gallagher G, Walker D, Wilson EM (1975) J Chem Soc Perk T 1:2030
Gallagher G (1978) US Patent 4,083,837
Eastlick DT (1978) US Patent 4,092,306
ter Wiel MKJ, Vicario J, Davey SG, Meetsma A, Feringa BL (2005) Org Biomol Chem 3:28
Nicolaou KC, Mathison CJN, Montagnon T (2004) J Am Chem Soc 126:5192
Weiss R, Seubert J (1994) Angew Chem Int Ed 33:891
Lapatsanis L, Milias G, Paraskewas S (1985) Synthesis 513
Furrow ME, Myers AG (2004) J Am Chem Soc 126:5436
Brewer M (2006) Tetrahedron Lett 47:7731
Justo de Pomar JC, Soderquist JA (2000) Tetrahedron Lett 41:3285
Javed MI, Brewer M (2008) Org Synth 85:189
Gassman PG, Greenlee WJ (1973) Org Synth 53:38
Rendina VL, Kingsbury JS (2012) J Org Chem 77:1181
Curtin DY, Gerber SM (1952) J Am Chem Soc 74:4052
Köpke T, Zaleski JM (2008) Anticancer Agents Med Chem 8:292
Lei X, Porco JA (2006) J Am Chem Soc 128:14790
Nicolaou KC, Li H, Nold AL, Pappo D, Lenzen A (2007) J Am Chem Soc 129:10356
Woo CM, Lu L, Gholap SL, Smith DR, Herzon SB (2010) J Am Chem Soc 132:2540
Herzon SB, Lu L, Woo CM, Gholap SL (2011) J Am Chem Soc 133:7260
Buchner E, Curtius T (1885) Ber Deut Bot Ges 18:2371
Staudinger H, Gaule A (1916) Ber Deut Bot Ges 49:1951
Nomura K, Iida T, Hori K, Yoshi E (1994) J Org Chem 59:488
Mahmood S, Hossain M (1998) J Org Chem 59:488
Kanemasa S, Kanai T, Araki T, Wada E (1999) Tetrahedron Lett 40:5055
Hashimoto T, Naganawa Y, Kano T, Maruoka K (2008) J Am Chem Soc 130:2434
Hashimoto T, Naganawa Y, Kano T, Maruoka K (2007) Chem Commun 5143
Hashimoto T, Miyamoto H, Naganawa Y, Maruoka K (2009) J Am Chem Soc 131:11280
Li W, Wang J, Hu X, Shen K, Wang W, Chu Y, Lin L, Liu X, Feng X (2010) J Am Chem Soc 132:8532
Gutsche CD, Hillman M (1954) J Am Chem Soc 76:2236
Kharasch MS, Rudy T, Nudenberg W, Büchi G (1953) J Org Chem 18:1030
Mock WL, Hartman ME (1970) J Am Chem Soc 92:5767
Mock WL, Hartman ME (1977) J Org Chem 42:459
Mock WL, Hartman ME (1977) J Org Chem 42:466
Liu HJ, Ogina T (1973) Tetrahedron Lett 14:4937
Liu HJ, Majumdar SP (1975) Synthetic Commun 5:125
Marchand AP, Annapurna P, Reddy SP, Watson WH, Nagl A (1989) J Org Chem 54:187
Dave V, Warnhoff EW (1983) J Org Chem 48:2590
Kantorowski EJ, Kurth MJ (2000) Tetrahedron 56:4317
Hashimoto T, Naganawa Y, Maruoka K (2009) J Am Chem Soc 131:6614
Nagao Y, Goto M, Ochiai M, Shiro M (1990) Chem Lett 19:1503
Baghdasarian G, Woerpel KA (2006) J Org Chem 71:6851
Hashimoto T, Naganawa Y, Maruoka K (2011) J Am Chem Soc 133:8834
Evans DA, Sweeney ZK, Rovis T, Tedrow JS (2001) J Am Chem Soc 123:12095
Evans DA, Scheidt KA, Fandrick KR, Lam HW, Wu J (2003) J Am Chem Soc 125:10780
Liu Y, Shang D, Zhou X, Liu X, Feng X (2009) Chem Eur J 15:2055
Ishikawa S, Hamada T, Manabe K, Kobayashi S (2004) J Am Chem Soc 126:12236
Bellemin-Laponnaz S, Gade LH (2002) Chem Commun 1286
Gade LH, Bellemin-Laponnaz S (2008) Chem Eur J 14:4142
Tee OS (1969) J Am Chem Soc 91:7144
Watkins SM, Reich A, Fleming LE, Hammond R (2008) Mar Drugs 6:431
Nicolaou KC, Yang Z, Shi G, Gunzner JL, Agrios KA, Gärtner P (1998) Nature 392:264
Mori Y, Yaegashi K, Furukawa H (1997) Tetrahedron 53:12917
Mori Y, Yaegashi K, Furukawa H (1997) J Am Chem Soc 119:4557
Mori Y, Nogami K, Hayashi H, Noyori R (2003) J Org Chem 68:9050
Pazos G, Pérez M, Gándara Z, Gómez G, Fall Y (2009) Tetrahedron Lett 50:5285
Seto H, Fujioka S, Koshino H, Hayasaka H, Shimizu T, Yoshida S, Watanabe T (1999) Tetrahedron Lett 40:2359
Smalley TL (2004) Synthetic Commun 34:1973
Drège E, Morgant G, Desmaële D (2005) Tetrahedron Lett 46:7263
Drège E, Tominiaux C, Morgant G, Desmaële D (2006) Eur J Org Chem 4825
Snyder SA, Wespe DA, von Hof JM (2011) J Am Chem Soc 133:8850
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Moebius, D.C., Rendina, V.L., Kingsbury, J.S. (2014). Catalysis of Diazoalkane–Carbonyl Homologation. How New Developments in Hydrazone Oxidation Enable the Carbon Insertion Strategy for Synthesis. In: Dong, G. (eds) C-C Bond Activation. Topics in Current Chemistry, vol 346. Springer, Berlin, Heidelberg. https://doi.org/10.1007/128_2013_521
Download citation
DOI: https://doi.org/10.1007/128_2013_521
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-55054-6
Online ISBN: 978-3-642-55055-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)