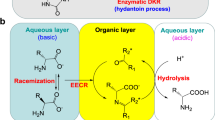
Abstract
Tanabe Seiyaku has been investigating an efficient optical resolution method for the productionof optically active amino acids since the 1950s. As one of the practical applications of the resolutionmethods, we focused on crystallization-induced asymmetric transformation, with which it is possibleto obtain more than 50% of one enantiomer of a racemate. In order to achieve the asymmetrictransformation, an elegant method for racemization of optically active amino acids and their saltswas developed. This successful racemization procedure led to efficient and economical preparationpaths for various optically active amino acids by the two crystallization-induced asymmetric transformations,one of which is a combination of enantiomeric resolution and simultaneous racemization and theother is a combination of diastereomeric resolution and simultaneous epimerization. Here, manyexamples of our studies and recent reports of pharmaceutical intermediates are presented.

Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Pasteur LM (1848) Campt Rend 26:535
Pasteur LM (1848) Ann Chem Phys [3]24:442
Jacques J, Collet A, Wilen SH (1981) Enantiomers, Racemates, and Resolutions. Wiley, New York
Eliel EL, Wilen SH, Mander LN (1994) Stereochemistry of Organic Compounds. Wiley, New York
Stinson SC (2001) Chemical & Engineering News, Vol. 79, 36. ACS, p 79
Rouhi AM (2003) Chemical & Engineering News, Vol. 81, 18. ACS, p 45
Rohhi AM (2004) Chemical & Engineering News, Vol. 82, 28. ACS, p 47
Collins AN, Sheldrake GN, Crosby J (1992) Chirality in Industry. Wiley, New York
Sheldon RA (1993) Chirotechnology. Marcel Dekker, New York
Collins AN, Sheldrake GN, Crosby J (1997) Chirality in Industry. Wiley, New York
Kurihara N, Miyamoto J (eds) (1998) Chirality in Agrochemicals. Wiley, New York
Ager DJ (ed) (1999) Handbook of Chiral Chemicals. Marcel Dekker, New York
Subramanian G (ed) (2001) Chiral Separation Techniques, a Practical Approach. Wiley, New York
RocSearch Ltd (2004) Chiral Technolgies: Market Potentialities and Technological Development
Yamada S, Hongo C, Chibata I (1980) Chem Ind (London) 5:539
Hongo C, Yamada S, Chibata I (1981) Bull Chem Soc Jpn 54:3286
Hongo C, Yamada S, Chibata I (1981) Bull Chem Soc Jpn 54:3291
Yamada S, Hongo C, Yoshioka R, Chibata I (1983) J Org Chem 48:843
Hongo C, Yoshioka R, Tohyama M, Yamada S, Chibata I (1983) Bull Chem Soc Jpn 56:3744
Hongo C, Yoshioka R, Tohyama M, Yamada S, Chibata I (1984) Bull Chem Soc Jpn 57:1328
Hongo C, Tohyama M, Yoshioka R, Yamada S, Chibata I (1985) Bull Chem Soc Jpn 58:433
Yoshioka R, Ohtsuki O, Tosa T (1986) Bunri Gijutsu 16:350
Yoshioka R, Tohyama M, Ohtsuki O, Yamada S, Chibata I (1987) Bull Chem Soc Jpn 60:649
Yoshioka R, Tohyama M, Yamada S, Ohtsuki O, Chibata I (1987) Bull Chem Soc Jpn 60:4321
Yoshioka R, Ohtsuki O, Senuma M, Tosa T (1989) Chem Pharm Bull 37:883
Buchanan C, Graham SH (1950) J Chem Soc, p 500
Harris MM (1958) Klyne W, Mare PBD (eds) Progress in Stereochemistry, vol 2. Butterworths Scientific Publications, London, p 157
McNaught AD, Wilkinson A (1997) IUPAC Compendium of Chemical Terminology, 2nd edn. Blackwell Science, Oxford
Suzuki K, Kiyooka S, Miyagawa T, Kawai A (1980) Nippon Kagaku Kaishi 2:287
Arai K (1986) J Synth Org Chem Jpn (Yuki Gosei Kagaku Kyokai Shi) 44:486
Dynamic kinetic resolution is occasionally confused with asymmetric transformation via crystallization. Alternatively, dynamic kinetic resolution via crystallization is rarely referred to as “Crystallization-induced dynamic resolution” [78,81]
Noyori R, Tokunaga M, Kitamura M (1995) Bull Chem Soc Jpn 68:36
Ward RS (1995) Tetrahedron: Asymmetr 6:1475
Caddick S, Jenkins K (1996) Chem Soc Rev, p 447
Faber K (2001) Chem Eur J 7:5004
Pellissier H (2003) Tetrahedron 59:8291
Kaneko T, Izumi Y, Chibata I, Itoh T (1974) Synthetic Production and Utilization of Amino Acids. Kodansha, Tokyo and Wiley, New York
Neuberger A (1948) In: Anson ML, Edsall JT (eds) Advance in Protein Chemistry, vol 4. Academic Press, New York, p 339
Sasaji I, Hara M, Tatsumi S, Seki K, Akashi T, Ohno K (1965) US Patent 3 213 106, Ajinomoto Co., Inc., Japan
Ogasawara H, Tatemichi H, Suzuki S (1967) JP Patent 42-13445
Metzler DE, Ikawa M, Snell EE (1954) J Am Chem Soc 76:648
Toi K, Izumi Y, Akabori S (1962) Bull Chem Soc Jpn 35:1422
Sakieki I, Mitsuno M (1959) J Chem Soc Chem Jpn 80:1035
Matsuo H, Kawazoe Y, Sato M, Ohnishi M, Tatsuno T (1970) Chem Pharm Bull 18:1788
Grigg R, Gunaratne HQN (1983) Tetrahedron Lett 24:4457
Chibata I, Yamada S, Yamamoto M, Wada M (1968) Experientia 24:638
Yamada S, Yamamoto M, Chibata I (1973) Chem Ind (London), (issue II), p 528
Yamada S, Yamamoto M, Chibata I (1973) J Agr Food Chem 21:889
Yamada S, Yamamoto M, Chibata I (1973) J Org Chem 38:4408
Yamada S, Yamamoto M, Hongo C, Chibata I (1975) J Agr Food Chem 23:653
Yamada S, Hongo C, Yamamoto M, Chibata I (1976) Agr Biol Chem 40:1425
Hongo C, Shibazaki M, Yamada S, Chibata I (1976) J Agr Food Chem 24:903
Hongo C, Yamada S, Chibata I (1981) Bull Chem Soc Jpn 54:1905
Hongo C, Yamada S, Chibata I (1981) Bull Chem Soc Jpn 54:1911
Yamada S, Hongo C, Chibata I (1978) Agr Biol Chem 42:1521
Yamada S, Hongo C, Chibata I (1977) Agr Biol Chem 41:2413
Yoshioka R, Ohtsuki O, Da-te T, Okamura K, Semuma M (1994) Bull Chem Soc Jpn 67:3012
Yamada S, Hongo C, Yoshioka R, Chibata I (1979) Agr Biol Chem 43:395
Yamada S, Yoshioka R, Shibatani T (1997) Chem Pharm Bull 45:1922
Yoshioka R, Okamura K, Yamada S, Aoe K, Da-te T (1998) Bull Chem Soc Jpn 71:1109
Yamada S, Tsujioka I, Shibatani T, Yoshioka R (1999) Chem Pharm Bull 47:146
Yoshioka R, Hiramatsu H, Okamura K, Tsujioka I, Yamada S (2000) J Chem Soc Perkin Trans 2, p 2121
Secor RM (1963) Chem Rev 63:297
Wilen SH, Collet A, Jacques J (1997) Tetrahedron 33:2725
Collet A, Brienne Mj, Jacques J (1980) Chem Rev 80:215
Boyle WJ Jr, Sifiniades S, Van Peppen JF (1979) J Org Chem 44:4841
Arai K, Obara Y, Iizumi T, Takakuwa Y (1985) 47th Yuki Gosei Symposium (Japanese), p 64
Takahashi Y, Arai K, Obara Y, Matsumoto H (1986) JP 61 103852 (Nissan Chemical Industries, Ltd., Japan)
Takahashi Y, Arai K, Obara Y, Matsumoto H (1986) Chem Abstr 105:114749t
Obara Y, Matsumoto H, Arai K, Tsuchiya S (1986) JP 61 1652 (Nissan Chemical Industries, Ltd., Japan)
Obara Y, Matsumoto H, Arai K, Tsuchiya S (1986) Chem Abstr 105:43327r
Okada Y, Takebayashi T, Sato S (1989) Chem Pharm Bull 37:5
Black SN, Williams LJ, Davey RJ, Moffatt F, Jones RVH, McEwan DM, Sadler DE (1989) Tetrahedron 45:2677
Nakano J, Taya N, Chaki H, Yamafuji T, Momonoi K (1994) JP 6-9615 (Toyama Chemical Co., Ltd., Japan)
Nakano J, Taya N, Chaki H, Yamafuji T, Momonoi K (1994) Chem Abstr 120:106751g
Murakami K, Aizawa N, Mochida S (1991) JP 91-56482 (Mochida Seiyaku, Co., Ltd., Japan)
Murakami K, Aizawa N, Mochida S (1991) Chem Abstr 115:56482q
Shieh W-C, Carlson JA (1994) J Org Chem 59:5463
Akashi T (1962) Nippon Kagaku Kaishi 83:421
Nohira H, Miura H (1975) Nippon Kagaku Kaishi 6:1122
Kozma D (2001) CRC Handbook of Optical Resolution via Diastereomeric Salt Formation. CRC, Boca Raton, Florida
Clark JC, Phillipps GH, Steer MR (1976) J Chem Soc Perkin Trans 1, p 475
Leuchs H, Wutke J (1913) Ber 46:2420
Betti M, Mayer M (1908) Ber 41:2071
Ingersoll AW (1925) J Am Chem Soc 47:1168
Wagatsuma M, Seto M, Miyagishima T, Kawazu M, Yamaguchi T, Ohshima S (1983) J Antibiot 36:147
Bhattacharya A, Araullo-Mcadams C, Meier MB (1994) Syn Commun 24:2449
Toda F, Tanaka K (1983) Chem Lett 661
Nicholson JS, Tantum JG (1987) JP 62-6536, DE 2 809 794 (Boots Co., Ltd.)
Nicholson JS, Tantum JG (1987) Chem Abstr 90:22610j
Shiraiwa T, Kataoka K, Sakata S, Kurokawa H (1989) Bull Chem Soc Jpn 62:109
Hassan NA, Bayer E, Jochims JC (1998) J Chem Soc Perkin Trans 1, p 3747
Maryanoff CA, Scott L, Shah RD, Villani FJ Jr (1998) Tetrahedron:Asymmetr 9:3247
Boesten WHJ, Seerden J-PG, de Lange B, Dielemants HJA, Elsenberg HLM, Kaptein B, Moody HM, Kellogg RM, Broxterman QB (2001) Org Lett 3:1121
Resnick L, Galante RJ (2006) Tetrahedron:Asymmetr 17:846
Jakubec P, Berkeš D, Považanec F (2004) Tetrahedron Lett 45:4755
Lee S-K, Lee SY, Park YS (2001) Synlett 1941
Berkeš D, Kolarovič A, Manduch R, Baran P, Považanec F (2005) Tetrahedron:Asymmetr 16:1927
Yamada M, Nagashima N, Hasegawa J, Takahashi S (1998) Tetrahedron Lett 39:9019
Caddick S, Jenkins K (1996) Tetrahedron Lett 37:1301
Brunetto G, Gori S, Fiaschi R, Napolitano E (2002) Helv Chem Acta 85:3785
Reider PJ, Davis P, Hughes DL, Grabowski EJJ (1987) J Org Chem 52:955
Kotera T, Watanabe M, Katori G, Ichihara M, Kkara K (1998) 63th Kagaku Kogaku Kai Nenkai (Japanese), p 123
Shi Y-J, Wells KM, Pye PJ, Choi W-B, Churchill HRO, Lynch JE, Maliakal A, Sager JW, Rossen K, Volante RP, Reider PJ (1999) Tetrahedron 55:909
Shieh W-C, Carlson JA, Zaunius GM (1997) J Org Chem 62:8271
Ikeura Y, Ishimaru T, Doi T, Kawada M, Fujishima A, Natsugari H (1998) Chem Commun 2141
Colson P-J, Przbyla CA, Wise BE, Babiak KA, Seaney LM, Korte DE (1998) Tetrahedron:Asmmetr 9:2587
Alabaster RJ, Gibson AW, Johnson SA, Edwards JS, Cottrell IF (1997) Tetrahedron:Asymmetr 8:447
Sohda T, Mizuno K, Kawamatsu Y (1984) Chem Pharm Bull 32:4460
Hagmann WK (1986) Syn Commun 16:437
Lopez FJ, Ferriňo SA, Reyes MS, Román R (1997) Tetraheron:Asymmetr 8:2497
Cannata V, Tamerlani G (1986) JP 61-129148, EP 182 279 (Alfa Chemicals Italiana S.p.A.)
Cannata V, Tamerlani G (1986) Chem Abstr 105:78693j
Cannata V, Tamerlani G, Morotti M (1986) JP 61-289057, EP 204 911 (Alfa Chemicals Italiana S.p.A.)
Cannata V, Tamerlani G, Morotti M (1987) Chem Abstr 107:6951k
Konoike T, Matsumura K, Yorifuji T, Shinomoto S, Ide Y, Ohya T (2003) J Org Chem 67:7741
Brands KMJ, Payack JF, Rosen JD, Nelson TD, Candelario A, Huffman MA, Zhao MM, Li J, Craig B, Song ZJ, Tschaen DM, Hansen K, Devine PN, Pye PJ, Rossen K, Dormer PG, Reamer RA, Welch CJ, Mathre Dj, Tsou NN, McNamara JM, Reider PJ (2003) J Am Chem Soc 125:2129
Silverberg LJ, Kelly S, Vemishetti P, Vipond DH, Gibson FS, Harrison B, Spector R, Dillon JL (2000) Org Lett 2:3281
Napolitano E, Farina V (2001) Tetrahedron Lett 42:3231
Ataka K, Okamura S (1997) JP 97 221 485 (Ube Industries, Ltd., Japan)
Ataka K, Okamura S (1997) Chem Abstr 127:248097s
Yokoyama Y (1998) Medicinal Chemistry Symposium No18 (Japanese), p 41
Cooper J, Humber DC, Long AG (1986) Syn Commun 16:1469
Kemperman GJ, Zhu J, Klunder AJH, Zwanenburg B (2000) Org Lett 2:2829
Yang KS (1981) JP 1-18077, EP 18 811 (Lilly, Eli, and Co.)
Yang KS (1981) Chem Abstr 94:180670r
Onoue H, Ohtani M, Watanabe F (1984) JP 59-007193, EP 98 545 (Shionogi and Co., Ltd.)
Onoue H, Ohtani M, Watanabe F (1984) Chem Abstr 101:23214r
Fuchs R, Wittig A (1987) JP 62-456, EP 206 149 (Bayer AG)
Fuchs R, Wittig A (1987) Chem Abstr 107:40134f
Takuma K, Suzuki Y, Morino H, Kakimizu A (1984) JP 58-183 662, EP 206 149 (Sumitomo Chemical Co., Ltd.)
Takuma K, Suzuki Y, Morino H, Kakimizu A (1984) Chem Abstr 100:116506g
Smrčina M, Lorenc M, Hanuš V, Sedmera P, Kočovsvský P (1992) J Org Chem 57:1917
Wilen SH, Qi JZ, Williard PG (1991) J Org Chem 56:485
Chaplin DA, Johnson NB, Paul JM, Potter GA (1998) Tetrahedron Lett 39:6777
Czollner L, Frantsits W, Küenburg B, Hedenig U, Fröhlich J, Jordis U (1998) Tetrahedron Lett 39:2087
Xia W, Scheffer JR, Patrick BO (2005) Cryst Eng Comm 7:728
Kanomata N, Ochiai Y (2001) Tetrahedron Lett 42:1045
Ates A, Curran DP (2001) J Am Chem Soc 123:5130
Ihara M, Taniguchi N, Yasui T, Hosoda S, Fukumoto K (1991) 33th Symposium on Natural Organic Compounds (Japanese), p 124
Vedejs E, Donde Y (2000) J Org Chem 65:2337
Node M, Nishide K, Fujiwara T, Ichihashi S (1998) Chem Commun 2363
Vedejs E, Donde Y (1997) J Am Chem Soc 119:9293
Komatsu H, Awano H (2002) J Org Chem 67:5419
Vedejs E, Chapman RW, Müller SLM, Powell DR (2000) J Am Chem Soc 122:3047
Allinger NL, Eliel EL (1971) Top Stereochem 6:107
Newman P (1978) Optical Resolution Procedures for Chemical Compounds, vol 1. Optical Resolution Information Center, New York
Newman P (1981) Optical Resolution Procedures for Chemical Compounds, vol 2. Optical Resolution Information Center, New York
Ebbers EJ, Ariaans GJA, Houbiers JPM, Bruggink A, Zwanenburg B (1997) Tetrahedron 53:9417
Faber K (1997) Biotransformations in Organic Chemistry, 3rd edn. Springer, Berlin Heidelberg New York
Strauss UT, Felfer U, Faber K (1999) Tetrahedron:Asymmetr 10:107
Fulling G, Sih CJ (1987) J Am Chem Soc 109:2845
van der Deen H, Cuiper AD, Hof RP, van Oeveren A, Feringa BL, Kellogg RM (1996) J Am Chem Soc 118:3801
Larsson ALE, Persson BA, Backvall J-E (1997) Angew Chem Int Ed Engl 36:1211
Strauss UT, Felfer U, Faber K (2005) Tetrahedron:Asymmetr 16:1927
Inoue Y (1992) Chem Rev 92:741
Leibovitch M, Olovsson G, Scheffer JR, Trotter J (1998) J Am Chem Soc 120:12755
Ramamurthy V, Schanze KS, Dekker M (1998) Molecular and Supramolecular Photochemistry, vol 2. Marcel Dekker, New York
Horspool WM, Lenci F (2004) CRC Handbook of Organic Photochemistry and Photobiology, 2nd edn. CRC, Boca Raton, FL
Author information
Authors and Affiliations
Corresponding author
Editor information
Rights and permissions
Copyright information
© 2007 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Yoshioka, R. (2007). Racemization, Optical Resolution and Crystallization-Induced Asymmetric Transformation of Amino Acids and Pharmaceutical Intermediates. In: Sakai, K., Hirayama, N., Tamura, R. (eds) Novel Optical Resolution Technologies. Topics in Current Chemistry, vol 269. Springer, Berlin, Heidelberg. https://doi.org/10.1007/128_2006_094
Download citation
DOI: https://doi.org/10.1007/128_2006_094
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-540-46317-7
Online ISBN: 978-3-540-46320-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)