Abstract
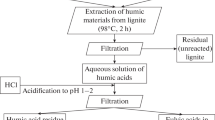
Humic and fulvic acids derived from South Ural lignite are studied. The composition of the coal, humic acids, and fulvic acids are characterized by technical and elemental analysis, Fourier transform infrared (FTIR) spectroscopy, and CP/MAS 13C NMR spectroscopy. A method is proposed for extracting fulvic acids from lignite.by means of an aqueous hydrochloric-acid solution of n-butanol. It is established that fulvic acids are characterized by higher content of oxygen-bearing aliphatic groups with a predominance of carboxylic acids and complex esters, while humic acids have a higher content of carbon, hydrogen, and aromatic fragments. Dynamic light scattering shows that colloidal aggregations of fulvic acids in water are disperse systems with bimodal (at low concentrations) and monomodal (at higher concentrations) size distributions of nanoparticles and submicroparticles. The low negative values of the zeta potential for aqueous fulvic acid colloids indicate instability and a tendency to complex formation.




Similar content being viewed by others
REFERENCES
Orlov, D.S., Gumusovye kisloty pochv i obshchaya teoriya gumifikatsii (Humic Acids of Soils and General Theory of Humification), Moscow: Izd-vo Mosk. Gos. Univ., 1990.
Orlov, D.S., Gumusovye kisloty pochv (Humic Acids of Soils), Moscow: Izd-vo Mosk. Gos. Univ., 1974.
Ruschev, D.D., Khimiya tverdogo topliva (Chemistry of Solid Fuel), Leningrad: Khimiya, 1976.
Zherebtsov, S.I., Malyshenko, N.V., Votolin, K.S., Ismagilov, Z.R., Androkhanov, V.A., Sokolov, D.A., and Dugarjav, J., Structural-group composition and biological activity of humic acids obtained from brown coals of Russia and Mongolia, Solid Fuel Chem., 2019, vol. 53, no. 3, pp. 145–151. https://doi.org/10.3103/S0361521919030121
Zherebtsov, S.I., Malyshenko, N.V., Votolin, K.S., Androkhanov, V.A., Sokolov, D.A., Dugarjav, J., and Ismagilov, Z.R., Study of the biological activity of humine substances for the creation of preparations against desertification, Chem. Sustainable Dev., 2019, vol. 27, no. 2, pp. 138–146. https://doi.org/10.15372/CSD2019121
Zherebtsov, S.I., Malyshenko, N.V., Votolin, K.S., Shpakodraev, K.M., and Ismagilov, Z.R., Investigation of the dependence of biological activity on the structural parameters of native and modified humic acids from brown coal, Khim. Interesakh Ustoich. Razvit., 2020, vol. 28, no. 2, pp. 152–158. https://doi.org/10.15372/KhUR2020213
Sokolov, D.A., Dobryanskaya, S.L., Androkhanov, V.A., Klekovkin, S.Yu., Gossen, I.N., Zherebtsov, S.I., Malyshenko, N.V., Votolin, K.S., and Dugarzhav, Zh., Assessment of the structural-group composition of humic acid from brown coals impact on their biological activity in conditions of technogenic landscapes, Vestn. Kuzbasskogo Gos. Tekh. Univ., 2018, no. 5, pp. 90–99. https://doi.org/10.26730/1999-4125-2018-5-90-99
Zherebtsov, S.I., Malyshenko, N.V., Votolin, K.S., and Ismagilov, Z.R., Sorption of metal cations by lignite and humic acids, Coke Chem., 2020, vol. 63, no. 3, pp. 142–148. https://doi.org/10.3103/S1068364X20030096
Zherebtsov, S.I., Malyshenko, N.V., Bryukhovets-kaya, L.V., Lyrshchikov, S.Yu., and Ismagilov, Z.R., Sorption of cobalt cations by humic acids, Coke Chem., 2018, vol. 61, no. 7, pp. 266–269. https://doi.org/10.3103/S1068364X18070086
Zherebtsov, S.I., Malyshenko, N.V., Bryukhovets-kaya, L.V., and Ismagilov, Z.R., Interaction of copper, zinc, and manganese cations with lignite and humic acids, Coke Chem., 2017, vol. 60, no. 10, pp. 397–403. https://doi.org/10.3103/S1068364X17100088
Klučáková, M., Size and charge evaluation of standard humic and fulvic acids as crucial factors to determine their environmental behavior and impact, Front. Chem., 2018, vol. 6, p. 235. https://doi.org/10.3389/fchem.2018.00235
Esfahani, M.R., Stretz, H.A., and Wells, M.J.M., Abiotic reversible self-assembly of fulvic and humic acid aggregates in low electrolytic conductivity solutions by dynamic light scattering and zeta potential investigation, Sci. Total Environ., 2015, vol. 537, pp. 81–92. https://doi.org/10.1016/j.scitotenv.2015.08.001
Yang, Y., Wu, S., and Huang, X., Experimental study on the effect of fulvic acid in waste slurry on flocculation and zeta potential, Sustainability, 2021, vol. 13, no. 14, p. 7784. https://doi.org/10.3390/su13147784
Uspenskaya E.V., Pleteneva T.V., Grebennikova T.V., Kazimova, I.V., Fedyakina, I.T., Lebedeva, V.V., Latyshev, O.E., Eliseeva, O.V., Larichev, V.F., Garaev, T.M., Maximova, T.V., Morozova, M.A., Hang, Ph.My, and Syroeshkin, A.V., A comprehensive biological and physico-chemical characterization of humic and fulvic acids nanoparticles as a perspective drug, Preprints, 2021, p. 2021060245. https://doi.org/10.20944/preprints202106.0245.v1
Palomino, D. and Stoll, S., Fulvic acids concentration and pH influence on the stability of hematite nanoparticles in aquatic systems, J. Nanopart. Res., 2013, vol. 15, p. 1428. https://doi.org/10.1007/s11051-013-1428-5
Palmer, N.E. and von Wandruszka, R., Dynamic light scattering measurements of particle size development in aqueous humic materials, Fresenius’ J. Anal. Chem., 2001, vol. 371, pp. 951–954. doi https://doi.org/10.1007/s002160101037
Baalousha, M., Motelica-Heino, M., and Le Coustumer, Ph., Conformation and size of humic substances: effects of major cation concentration and type, pH, salinity, and residence time, Colloids Surf., A, 2006, vol. 272, nos. 1–2, pp. 48–55. https://doi.org/10.1016/j.colsurfa.2005.07.010
Votolin, K.S., Zherebtsov, S.I., Shpakodraev, K.M., Malyshenko, N.V., and Ismagilov, Z.R., Composition of humic and fulvic acids from lignite, Coke Chem., 2022, vol. 65, no. 5, pp. 191–200. https://doi.org/10.3103/S1068364X22050040
GOST (State Standard) 33503-2015: Solid mineral fuel. Methods for determination of moisture in the analysis sample, 2017.
GOST R (State Standard) 55661-2013: Solid mineral fuel. Determination of ash, 2015.
GOST R (State Standard) 55660-2013: Solid mineral fuel. Determination of volatile matter, 2015.
GOST (State Standard) 2408.1-95 (ISO 625-96): Solid fuel. Methods for determination of carbon and hydrogen, 1997.
Bellamy, L.J., The Infrared Spectra of Complex Molecules, London: Wiley, 1954.
Nakanishi, K., Infrared Spectra and Structure of Organic Compounds, Holden-Day, 1957.
ISO—International Organization for Standardization. https://www.iso.org/obp/ui/#iso:std:iso:22412:ed-1:v1:ru.
Efimova, O.S., Kolmykov, R.P., Panina, L.V., Dudnikova, Yu.N., and Ismagilov, Z.R., Determining the composition and particle size of coal dust by dynamic light scattering, Coke Chem., 2021, vol. 64, no. 11, pp. 488–495. https://doi.org/10.3103/S1068364X2111003X
Efimova, O.S., Fedorova, N.I., Sozinov, S.A., and Ismagilov, Z.R., Chemical and granulometric composition of coal dust from a mine degasing installation, Khim. Interesakh Ustoich. Razvit., 2018, vol. 26, no. 6, pp. 597–602. https://doi.org/10.15372/KhUR20180605
Aronov, C.G. and Nesterenko, L.L., Khimiya tverdykh goryuchikh iskopaemykh (Chemistry of Solid Combustible Fossils), Kharkov: Izd-vo Khar’kovskogo Univ., 1960.
Rice, J.A. and MacCarthy, P., Statistical evaluation of the elemental composition of humic substances, Org. Geochem., 1991, vol. 17, no. 5, pp. 635–648. https://doi.org/10.1016/0146-6380(91)90006-6
Rus’yanova, N.D., Uglekhimiya (Carbon Chemistry), Moscow: Nauka, 2003.
ACKNOWLEDGMENTS
The research was conducted on equipment at the Collective-Use Center, Federal Research Center of Coal and Coal Chemistry, Siberian Branch, Russian Academy of Sciences.
Funding
Financial support was provided by the Institute of Coal Chemistry and Materials Science, Federal Research Center of Coal and Coal Chemistry, Siberian Branch, Russian Academy of Sciences (project 121031500124-2).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by B. Gilbert
About this article
Cite this article
Votolin, K.S., Efimova, O.S., Zherebtsov, S.I. et al. Lignite Fulvic Acids: Analysis by Dynamic Light Scattering. Coke Chem. 65, 363–370 (2022). https://doi.org/10.3103/S1068364X22700016
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068364X22700016