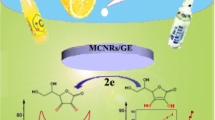
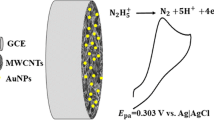
Abstract
A novel amperometric sensor for uric acid based on ordered mesoporous carbon modified pyrolytic graphite electrode was developed. Uric acid oxidation was easily catalyzed by this electrode in a phosphate buffer solution at pH 7.0, with an anodic potential decrease about 140 mV compared to bare pyrolytic graphite electrode. The uric acid level was determined by the amperometric method, at a constant potential of 0.31 mV, the catalytic current of uric acid vs. its concentration showed a good linearity in the range of 1.0 × 10−6−1.0 × 10−4 mol L−1, with a correlation coefficient of 0.999. The detection limit was 4.0 × 10−7 mol L−1. The proposed method could be effectively used for uric acid amperometric sensing in human urine.
Similar content being viewed by others
References
Ardakani, M. M., Akrami, Z., Kazemian, H., & Zare, H. R. (2006). Electrocatalytic characteristics of uric acid oxidation at graphite-zeolite-modified electrode doped with iron(III). Journal of Electroanalytical Chemistry, 586, 31–38. DOI: 10.1016/j.jelechem.2005.09.015.
Cunningham, S. K., & Keaveny, T. V. (1978). A two-stage enzymatic method for determination of uric acid and hypoxanthine/xanthine. Clinica Chimica Acta, 86, 217–221. DOI: 10.1016/0009-8981(78)90135-3.
Czauderna, M., & Kowalczyk, J. (2000). Quantification of allantoin, uric acid, xanthine and hypoxanthine in ovine urine by high-performance liquid chromatography and photodiode array detection. Journal of Chromatography B, 744, 129–138. DOI: 10.1016/S0378-4347(00)00239-5.
Davis, M. E. (2002). Ordered porous materials for emerging applications. Nature, 417, 813–821. DOI: 10.1038/nature00785.
Ferraris, S. P., Lew, H., & Elsayed, N. M. (1991). Simultaneous determination of inosine, hypoxanthine, xanthine, and uric acid and the effect of metal chelators. Analytical Biochemistry, 195, 116–121. DOI: 10.1016/0003-2697(91)90305-D.
Filisetti-Cozzi, T. M., & Carpita, N. C. (1991). Measurement of uronic acids without interference from neutral sugars. Analytical Biochemistry, 197, 157–162. DOI: 10.1016/0003-2697(91)90372-Z.
Hu, G., Ma, Y., Guo, Y., & Shao, S. (2008). Electrocatalytic oxidation and simultaneous determination of uric acid and ascorbic acid on the gold nanoparticles-modified glassy carbon electrode. Electrochimica Acta, 53, 6610–6615. DOI: 10.1016/j.electacta.2008.04.054.
Jia, N., Wang, Z., Yang, G., Shen, H., & Zhu, L. (2007). Electrochemical properties of ordered mesoporous carbon and its electroanalytical application for selective determination of dopamine. Electrochemistry Communications, 9, 233–238. DOI: 10.1016/j.elecom.2006.08.050.
Jin, W., Li, T.-H., Wang, X.-X., Ji, Y.-B., & Li, X.-T. (2007). Synthesis of ordered mesoporous carbon based on evaporation-induced self-assembly method. Carbon Techniques, 26(6), 16–20.
Joo, S. H., Choi, S. J., Oh, I., Kwak, J., Liu, Z., Terasaki, O., & Ryoo, R. (2001). Ordered nanoporous arrays of carbon supporting high dispersions of platinum nanoparticles. Nature, 412, 169–172. DOI: 10.1038/35084046.
Jun, S., Joo, S. H., Ryoo, R., Kruk, M., Jaroniec, M., Liu, Z., Ohsuna, T., & Terasaki, O. (2000). Synthesis of new, nanoporous carbon with hexagonally ordered mesostructure. Journal of the American Chemical Society, 122, 10712–10713. DOI: 10.1021/ja002261e.
Kalimuthu, P., Suresh, D., & John, S. A. (2006). Uric acid determination in the presence of ascorbic acid using self-assembled submonolayer of dimercaptothiadiazole-modified gold electrodes. Analytical Biochemistry, 357, 188–193. DOI: 10.1016/j.ab.2006.07.031.
Kennedy, L. J., Vijaya, J. J., Kayalvizhi, K., & Sekaran, G. (2007). Adsorption of phenol from aqueous solutions using mesoporous carbon prepared by two-stage process. Chemical Engineering Journal, 132, 279–287. DOI: 10.1016/j.cej.2007.01.009.
Li, Z., Feng, M., & Lu, J. (1998). KMnO4-octylphenyl polygylcol, ether chemiluminescence system for flow injection analysis of uric acid in urine. Microchemical Journal, 59, 278–283. DOI: 10.1006/mchj.1997.1537.
Lima, P. R., Santos, W. J. R., Oliveira, A. B., Goulart, M. O. F., & Kubota, L. T. (2008). Electrocatalytic activity of 4-nitrophthalonitrile-modified electrode for the L-glutathione detection. Journal of Pharmaceutical and Biomedical Analysis, 47, 758–764. DOI: 10.1016/j.jpba.2008.03.006.
Ndamanisha, J. C., & Guo, L. (2008). Electrochemical determination of uric acid at ordered mesoporous carbon functionalized with ferrocenecarboxylic acid-modified electrode. Biosensors and Bioelectronics, 23, 1680–1685. DOI: 10.1016/j.bios.2008.01.026.
Pournaghi-Azar, M. H., & Saadatirad, A. (2008). Simultaneous voltammetric and amperometric determination of morphine and codeine using a chemically modified-palladized aluminum electrode. Journal of Electroanalytical Chemistry, 624, 293–298. DOI: 10.1016/j.jelechem.2008.09.016.
Wang, G., Meng, J., Liu, H., Jiao, S., Zhang, W., Chen, D., & Fang, B. (2008). Determination of uric acid in the presence of ascorbic acid with hexacyanoferrate lanthanum film modified electrode. Electrochimica Acta, 53, 2837–2843. DOI: 10.1016/j.electacta.2007.10.064.
Yuan, X., Xing, W., Zhuo, S.-P., Si, W., Gao, X., Han, Z., & Yan, Z.-F. (2008). Adsorption of bulky molecules of nonylphenol ethoxylate on ordered mesoporous carbons. Journal of Colloid and Interface Science, 322, 558–565. DOI: 10.1016/j.jcis.2008.02.032.
Zen, J.-M., Jou, J.-J., & Ilangovan, G. (1998). Selective voltammetric method for uric acid detection using pre-anodized Nafion-coated glassy carbon electrodes. Analyst, 123, 1345–1350. DOI: 10.1039/a801532e.
Zhang, Y., Wen, G., Zhou, Y., Shuang, S., Dong, C., & Choi, M. M. F. (2007). Development and analytical application of an uric acid biosensor using an uricase-immobilized eggshell membrane. Biosensors and Bioelectronics, 22, 1791–1797. DOI: 10.1016/j.bios.2006.08.038.
Zhou, H., Zhu, S., Hibino, M., Honma, I., & Ichihara, M. (2003). Lithium storage in ordered mesoporous carbon (CMK-3) with high reversible specific energy capacity and good cycling performance. Advanced Materials, 15, 2107–2111. DOI: 10.1002/adma.200306125.
Zhou, M., Ding, J., Guo, L.-P., & Shang, Q.-K. (2007). Electrochemical behavior of L-cysteine and its detection at ordered mesoporous carbon-modified glassy carbon electrode. Analytical Chemistry, 79, 5328–5335 DOI: 10.1021/ac0703707.
Zhu, L., Tian, C., Zhu, D., & Yang, R. (2008). Ordered mesoporous carbon paste electrodes for electrochemical sensing and biosensing. Electroanalysis, 20, 1128–1134. DOI: 10.1002/elan.200704162.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, Y., Hu, G., Shao, S. et al. An amperometric sensor for uric acid based on ordered mesoporous carbon-modified pyrolytic graphite electrode. Chem. Pap. 63, 641–645 (2009). https://doi.org/10.2478/s11696-009-0076-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-009-0076-9