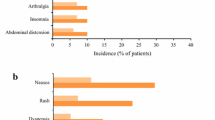
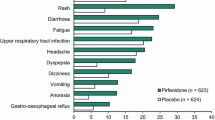
Abstract
Pirfenidone is an orally administered pyridine that has orphan designation for the treatment of mild to moderate idiopathic pulmonary fibrosis (IPF) in the EU.
Pirfenidone 2403 mg/day for 72 weeks administered to patients with IPF was associated with a significantly lower mean decline in the percent predicted forced vital capacity than placebo (primary endpoint) according to data from one of two randomized, double-blind, multinational trials (studies 004 and 006 [also known as the CAPACITY trials]), and data from a pooled analysis of both trials.
In another randomized, double-blind, multicentre Japanese trial, the adjusted mean in the change in vital capacity from baseline to week 52 was significantly lower in patients with IPF who received pirfenidone 1800 mg/day (considered to be comparable to the 2403 mg/day dose in studies 004 and 006 on a weight-normalized basis) than in those who received placebo (primary endpoint).
Pirfenidone had an acceptable tolerability profile in clinical trials, with most adverse events being mild to moderate in severity.








Similar content being viewed by others
References
Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011 Mar 15; 183(6): 788–824
Hauber H-P, Blaukovitsch M. Current and future treatment options in idiopathic pulmonary fibrosis. Inflamm Allergy Drug Targets 2010 Jul 1; 9(3): 158–72
European Medicines Agency. Public summary of opinion on orphan designation: pirfenidone for the treatment of idiopathic pulmonary fibrosis (EMA/COMP/198295/2004 Rev. 2) [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/Orphan_designation/2009/10/WC500006133.pdf [Accessed 2011 Jun 7]
Bouros D. Pirfenidone for idiopathic pulmonary fibrosis [letter]. Lancet 2011 May 21; 377(9779): 1727–9
InPharm. First idiopathic pulmonary fibrosis drug approved [online]. Available from URL: http://www.inpharm.com/news/149931/intermune-idiopathic-pulmonaryfibrosis-esbriet-pirfenidone [Accessed 2011 Jul 12]
Macías-Barragán J, Sandoval-Rodríguez A, Navarro-Partida J, et al. The multifaceted role of pirfenidone and its novel targets. Fibrogenesis Tissue Repair 2010; 3: 16
Jackson RM, Gomez-Marin O. Development and utility of pirfenidone in the treatment of idiopathic pulmonary fibrosis: review of preclinical science and recent clinical trials. Transpl Res Risk Manag 2011; 3: 55–63
Oku H, Shimizu T, Kawabata T, et al. Antifibrotic action of pirfenidone and prednisolone: different effects on pulmonary cytokines and growth factors in bleomycin-induced murine pulmonary fibrosis. Eur J Pharmacol 2008 Aug 20; 590 (1-3): 400–8
Iyer SN, Gurujeyalakshmi G, Giri SN. Effects of pirfenidone on transforming growth factor-b gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharmacol Exp Ther 1999 Oct; 291(1): 367–73
European Medicines Agency. Esbriet®: CHMP assessment report (EMA/CHMP/115147/2011) [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002154/WC500103073.pdf [Accessed 2011 May 1]
Gurujeyalakshmi G, Hollinger MA, Giri SN, et al. Pirfenidone inhibits PDGF isoforms in bleomycin hamster model of lung fibrosis at the translational level. Am J Physiol Lung Cell Mol Physiol 1999 Feb 1; 276(2): L311–8
Iyer SN, Gurujeyalakshmi G, Giri SN. Effects of pirfenidone on procollagen gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharmacol Exp Ther 1999 Apr; 289(1): 211–8
Iyer SN, Wild JS, Schiedt MJ, et al. Dietary intake of pirfenidone ameliorates bleomycin-induced lung fibrosis in hamsters. J Labs Clin Med 1995; 125: 779–85
Schaefer CJ, Ruhrmund DW, Pan L, et al. Antifibrotic activities of pirfenidone in animal models. Eur Respir Rev 2011 Jun 1; 20(120): 85–97
Kakugawa T, Mukae H, Hayashi T, et al. Pirfenidone attenuates expression of HSP47 in murine bleomycin-induced pulmonary fibrosis. Eur Respir J 2004 Jul; 24(1): 57–65
Iyer SN, Hyde DM, Giri SN. Anti-inflammatory effect of pirfenidone in the bleomycin-hamster model of lung inflammation. Inflammation 2000 Oct; 24(5): 477–91
Spond J, Case N, Chapman RW, et al. Inhibition of experimental acute pulmonary inflammation by pirfenidone. Pulm Pharmacol Ther 2003 Aug; 16(4): 207–14
Oku H, Nakazato H, Horikawa T, et al. Pirfenidone suppresses tumor necrosis factor-α, enhances interleukin-10 and protects mice from endotoxic shock. Eur J Pharmacol 2002 Jun 20; 446(1-3): 167–76
Nakazato H, Oku H, Yamane S, et al. A novel anti-fibrotic agent pirfenidone suppresses tumor necrosis factor-α at the translational level. Eur J Pharmacol 2002 Jun 20; 446(1–3): 177–85
Koyama S, Kamijyo S, Hashizume M, et al. Pirfenidone attenuates the release of neutrophil (NCA) and monocyte chemotactic activity (MCA) from human lung fibroblasts (FLH-1) in response to tumor necrosis factor (TNF)-alpha [abstract]. Am J Respir Crit Care Med 2010 May 1; 181 Suppl.: A3529
Misra H, Rabideau C. Pirfenidone inhibits NADPH-dependent microsomal lipid peroxidation and scavenges hydroxyl radicals. Molec Cell Biochem 2000 Jan; 204(1–2): 119–26
Giri SN, Leonard S, Shi X, et al. Effects of pirfenidone on the generation of reactive oxygen species in vitro. J Environ Pathol Toxicol Oncol 1999; 18(3): 169–77
Mitani Y, Sato K, Muramoto Y, et al. Superoxide scavenging activity of pirfenidone-iron complex. Biochem Biophys Res Commun 2008 Jul; 372(1): 19–23
Kaneko M, Inoue H, Nakazawa R, et al. Pirfenidone induces intercellular adhesion molecule-1 (ICAM-1) down-regulation on cultured human synovial fibroblasts. Clin Exp Immunol 1998; 113: 72–6
Shi S, Wu J, Chen H, et al. Single-and multiple-dose pharmacokinetics of pirfenidone, an antifibrotic agent, in healthy Chinese volunteers. J Clin Pharmacol 2007 Oct; 47(10): 1268–76
Rubino CM, Bhavnani SM, Ambrose PG, et al. Effect of food and antacids on the pharmacokinetics of pirfenidone in older healthy adults. Pulm Pharmacol Ther 2009 Aug; 22(4): 279–85
InterMune. Esbriet® (pirfenidone): EU summary of product characteristics [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002154/WC500103049.pdf [Accessed 2011 May 11]
Raghu G, Johnson WC, Lockhart D, et al. Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, pirfenidone: results of a prospective, open-label phase II study. Am J Respir Crit Care Med 1999 Apr 1; 159(4): 1061–9
Nagai S, Hamada K, Shigematsu M, et al. Open-label compassionate use one year-treatment with pirfenidone to patients with chronic pulmonary fibrosis. Intern Med 2002 Dec; 41(12): 1118–23
Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2005 May 1; 171(9): 1040–7
Noble PW, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 2011; 377(9779): 1760–9
Taniguchi H, Ebina M, Kondoh Y, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J 2010 Apr; 35(4): 821–9
Costabel U, Albera C, Bradford W, et al. Pirfenidone dose-response in patients with idiopathic pulmonary fibrosis (IPF): a comprehensive analysis of outcomes in CAPACITY 2 [abstract no. 388]. 20th Annual Congress of the European Respiratory Society; 2010 Sep 18–22; Barcelona
Valeyre D, Albera C, Bradford W, et al. The effect of treatment with pirfenidone on death or lung transplantation in patients with idiopathic pulmonary fibrosis (IPF): analysis of outcomes in the CAPACITY (CAP) trials [abstract no. 391]. 20th Annual Congress of the European Respiratory Society; 2010 Sep 18–22; Barcelona
Ley B, Collard HR, King TE. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011 Feb; 183(4): 431–40
Albera C, Bradford W, Costabel U, et al. The magnitude of pirfenidone treatment effect in patients with idiopathic pulmonary fibrosis (IPF): a pooled analysis of outcomes in the CAPACITY (CAP) studies [abstract no. 389]. 20th Annual Congress of the European Respiratory Society; 2010 Sep 18–22; Barcelona
ClinicalTrials.gov. Efficacy and safety of pirfenidone in patients with idiopathic pulmonary fibrosis (IPF) (ASCEND): NCT01366209 [online]. Available from URL: http://clinicaltrials.gov/ct2/show/NCT01366209?term=pirfenidone&rank=4 [Accessed 2011 Jun 16]
InterMune. InterMune to provide overview of corporate strategy, update on Esbriet® commercialization plans in the EU and details of phase 3 ASCEND study [online]. Available from URL: http://phx.corporate-ir.net/phoenix.zhtml?c=100067&p=irol-newsArticle&ID=1567821&highlight= [Accessed 2011 Jun 16]
Acknowledgements and Disclosures
The manuscript was reviewed by: D. Bouros, Department of Pneumonology, University Hospital and Medical School Democritus University of Thrace, Alexandroupolis, Greece; J. Egan, Heart and Lung Transplant Programme, Mater Misericordiae Hospital, Dublin, Ireland; T. Nukiwa, Department of Respiratory Medicine, Ohoku University Graduate School of Medicine, Sendai, Japan.
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was also offered an opportunity to comment on this article. Changes resulting from comments received were made by the author on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carter, N.J. Pirfenidone. Drugs 71, 1721–1732 (2011). https://doi.org/10.2165/11207710-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11207710-000000000-00000