Abstract
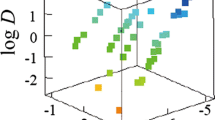
The stability constants and the hydration number of a complex between tris(pivaloyltrifluoroacetonato)lanthanide(III) (LnA3) and 2,2′-bipyridyl (B), LnA3B, and the complexation heat were determined across the Ln series by a solvent–extraction technique, Karl Fischer coulometry, and calorimetry, successively. The number of water molecules released from LnA3 upon the complexation with B as well as the values of the stability constant increased along with increasing Ln atomic number to around Dy(III); via a maximum, it decreased. It is concluded that the largest stability constant of the complex of Ho(III) or this vicinity is derived from the strongest bond energy, due to the fitness between LnA3 and B. This conclusion was supported by the trend of the variation of the entropy change as well as that of the hydration number across the Ln series.
Similar content being viewed by others
References
T. Sekine and D. Dyrssen, J. Inorg. Nucl. Chem., 1967, 29, 1481.
L. Farbu, J. Alstad, and J. H. Augustson, J. Inorg. Nucl. Chem., 1974, 36, 2091.
T. Shigematsu, M. Tabushi, M. Matsui, and T. Honjo, Bull. Chem. Soc. Jpn., 1967, 40, 2807.
Y. Hasegawa, T. Yamada, and K. Nagata, Solvent Extr. Ion Exch., 1996, 14, 89.
Y. Hasegawa, T. Ohyama, and S. Katsuta, Bull. Chem. Soc. Jpn., 1995, 68, 3091.
I. Matsubayashi, E. Ishiwata, T. Shionoya, and Y. Hasegawa, Talanta, 2004, 63, 625.
A. S. Kertes and E. E. Kassierer, Inorg. Chem., 1972, 11, 2108.
S. Nakamura and N. Suzuki, Inorg. Chim. Acta, 1986, 114, 101.
S. Nakamura and N. Suzuki, Polyhedron, 1988, 7, 155.
S. Yajima and Y. Hasegawa, Bull. Chem. Soc. Jpn., 1998, 71, 2825.
T. Ito and Y. Hasegawa, Solvent Extr. Res. Dev., Jpn., 2001, 8, 47.
Y. Hasegawa, M. Miratsu, and T. Kondo, Inorg. Chim. Acta, 2000, 303, 291.
Y. Hasegawa, M. Miratsu, and G. R. Choppin, Anal. Chim. Acta, 2001, 428, 149.
T. Sekine, Y. Hasegawa, and N. Ihara, J. Inorg. Nucl. Chem., 1973, 35, 3968.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saito, T., Sato, K., Miratsu, M. et al. Several Factors Influencing the Stability of Extracted Species in Synergistic Extraction of Lanthanide(III) with Pivaloyltrifluoro acetone and 2,2′-Bipyridyl. ANAL. SCI. 26, 1187–1191 (2010). https://doi.org/10.2116/analsci.26.1187
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2116/analsci.26.1187