Abstract
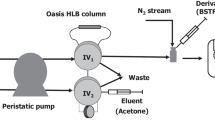
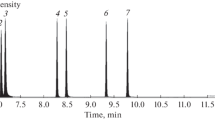
Nanomolar concentrations of NH2OH in natural water sources were determined using an Fe3+ oxidation method. A pH of 2.35–2.50 was used, which was adjusted by adding a chloroacetate buffer. Equal amounts (1.0 mL) of the chloroacetate solution and ferric chloride solution were added to the water sample (70 mL) to oxidize NH2OH to N2O. The resulting N2O in the sample water was then quantified by headspace analysis using a gas chromatograph with an electron-capture detector (ECD), where a limit of detection of 0.2 μgN L–1 (14 nmol L–1) was achieved. This method was successfully applied to samples of freshwater, brackish water, and seawater, and despite the various salinities no interfering substances were observed. Furthermore, NH2OH was successfully detected in samples collected from the Hii River and Lakes Shinji and Nakaumi (Shimane Prefecture, Japan). In addition, the proposed method was also applicable to samples rich in organic substance derived from phytoplankton.
Similar content being viewed by others
References
Y. Seike, K. Kondo, H. Hashitani, M. Okumura, K. Fujinaga, and Y. Date, Jpn. J. Limnol., 1990, 57, 137.
Y. Senga, Y. Seike, K. Mochida, K. Fujinaga, and M. Okumura, Limnology, 2001, 2, 129.
Y. Senga, K. Mochida, N. Okamoto, R. Fukumori, and Y. Seike, Limnology, 2002, 3, 21.
T. Hofman and H. Lees, Biochem. J., 1952, 54, 579.
M. Tanaka, Nature, 1953, 171, 1160.
M. Oshiki, M. Ali, K. Shinyako-Hata, H. Satoh, and S. Okabe, Environ. Microbiol., 2016, 18, 3133.
K. Kobayashi, A. Makabe, M. Yano, M. Oshiki, T. Kindaichi, K. L. Casciotti, and S. Okabe, ISME J., 2019, 73, 2426.
B. Kartal and T. Keltjens, Trends Biochem. Sci., 2016, 41, 998.
M. Fiadeiro, L. Solorzano, and J. D. H. Strickland, Limnol. Oceanogr., 1967, 12, 555.
J. H. Anderson, Analyst, 1964, 89, 357.
L. R. Pittwell, Microchim. Acta., 1975, 64, 425.
R. Fukumori, Y. Senga, M. Okumura, K. Fujinaga, and Y. Seike, Bunseki Kagaku, 2003, 52, 747.
A. Afkhami, T. Madrakian, and A. Maleki, Anal. Sci., 2006, 22, 329.
W. D. Korte, J. Chromatogr., 1992, 603, 145.
Y. Seike, R. Fukumori, Y. Senga, H. Oka, K. Fujinaga, and M. Okumura, Anal. Sci., 2004, 20, 139.
T. Kato, S. Sugahara, M. Murakami, Y. Senga, M. Egawa, H. Kamiya, K. Omata, and Y. Seike, Anal. Sci., 2017, 33, 691.
M. T.. von Breymann, M. A.. de Angells, and L. I. Gordon, Anal. Chem., 1982, 54, 1209.
J. H. Butler and L. I. Gordon, Mar. Chem., 1986, 19, 229.
J. P. Riley and G. Skirrows, “Chemical Oceanography”, 1965, Academic Press, New York, 648.
Y. Senga, K. Mochida, R. Fukumori, N. Okamoto, and Y. Seike, Estuarine Coastal Shelf Science, 2006, 67, 231.
R. F. Weiss and B. A. Price, Mar. Chem., 1980, 8, 347.
Acknowledgments
Parts of this study were performed with support from the Ministry of Education, Culture, Sports, Science and Technology Grant-in-Aid for Scientific Research (No. 19K22910). We would like to thank Editage (www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hikino, A., Sugahara, S., Kato, T. et al. Sensitive Gas Chromatography Detection of Nanomolar Hydroxylamine in Environmental Water by Fe(III) Oxidation. ANAL. SCI. 37, 347–351 (2021). https://doi.org/10.2116/analsci.20P254
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2116/analsci.20P254