Abstract
Background
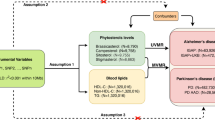
To investigate the causal relationship between testosterone (BT) levels and Alzheimer’s disease (AD) risk and to quantify the role of obesity and lipid metabolism as potential mediators.
Methods
We used a two-sample, two-step MR to determine:1) the causal effect of BT levels on AD; 2) the causal effect of two lipid metabolites, obesity and LDLc on AD; and 3) the mediating effects of these metabolites. Pooled data for BT levels and lipid metabolism were obtained from the UK Biobank. AD data were obtained from the Alzheimer’s Disease Project International Genomics Consortium, FinnGen Consortium, and UK Biobank study. Effect estimates from external genome-wide association study (GWAS) pooled statistics were obtained using inverse variance-weighted (IVW) MR analysis.
Results
Higher levels of BT were associated with a reduced risk of AD (odds ratio [OR] 0.9992, 95% CI 0.9985–0.9998, P = 0.019), and there was a negative correlation with LDLc (OR 0.9208, 95% CI 0.8569–0.9895, P = 0.024) and obesity class 2 (OC2) (OR 0.7445, 95% CI 0.5873–0.9437, P = 0.014). Conversely, there was a positive correlation between LDLc (OR 1.0014, 95% CI 1.0000–1.0029, P = 0.043) and OC2 (OR 1.0005, 95% CI 1.0001–1.0009, P = 0.003) and AD. Mediation analysis showed that the indirect effect of BT levels on AD was achieved through LDLc and OC2, which accounted for 17% and 17% of the total effect, respectively.
Conclusion
Our study identified a causal role of BT levels in LDLc and OC2. BT levels may affect AD through LDLc and OC2 metabolic processes.




Similar content being viewed by others
Availability of data and materials: Data from the IEU OpenGWAS project (https://gwas.mrcieu.ac.uk/) are available for researchers to apply to individual cohorts through their website. All other data used are publicly available and cited according in the text.
Abbreviations
- AD:
-
Alzheimer’s disease
- BT:
-
Bioavailable testosterone
- MR:
-
Mendelian randomization
- GWAS:
-
Genome-wide association study
- IVW:
-
Inverse variance-weighted
- OR:
-
Odds ratio
- LDLc:
-
LDL cholesterol
- OC2:
-
Obesity class 2
- Aβ:
-
β-amyloid
- NHANES data:
-
National Health and Nutrition Examination Survey data
- SNPs:
-
Single Nucleotide Polymorphisms
- STROBE-MR:
-
Strengthening the Reporting of Observational Studies in Epidemiology-Mendelian Randomization
- MVMR:
-
Multivariable Mendelian randomiza-tion
- FTS:
-
F-statistic
References
Knopman DS, Amieva H, Petersen RC, Chételat G, Holtzman DM, Hyman BT, et al. Alzheimer disease. Nat Rev Dis Primer. 2021;7: 33. DOI: https://doi.org/10.1038/s41572-021-00269-y
2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020;16: 391–460. DOI: https://doi.org/10.1002/alz.12068
Pike CJ. Sex and the development of Alzheimer’s disease: Sex Differences in AD. J Neurosci Res. 2017;95: 671–680. DOI: https://doi.org/10.1002/jnr.23827
Camacho EM, Huhtaniemi IT, O’Neill TW, Finn JD, Pye SR, Lee DM, et al. Age-associated changes in hypothalamic–pituitary–testicular function in middle-aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European Male Ageing Study. Eur J Endocrinol. 2013;168: 445–455. DOI: https://doi.org/10.1530/EJE-12-0890
Shi Z, Araujo AB, Martin S, O’Loughlin P, Wittert GA. Longitudinal Changes in Testosterone Over Five Years in Community-Dwelling Men. J Clin Endocrinol Metab. 2013;98: 3289–3297. DOI: https://doi.org/10.1210/jc.2012-3842
Marriott RJ, Murray K, Flicker L, Hankey GJ, Matsumoto AM, Dwivedi G, et al. Lower serum testosterone concentrations are associated with a higher incidence of dementia in men: The UK Biobank prospective cohort study. Alzheimers Dement. 2022;18: 1907–1918. DOI: https://doi.org/10.1002/alz.12529
LeBlanc ES, Wang PY, Janowsky JS, Neiss MB, Fink HA, Yaffe K, et al. Association between sex steroids and cognition in elderly men. Clin Endocrinol (Oxf). 2010;72: 393–403. DOI: https://doi.org/10.1111/j.1365-2265.2009.03692.x
Wang L, Pei J-H, Jia J-X, Wang J, Song W, Fang X, et al. Inhibition of oxidative stress by testosterone improves synaptic plasticity in senescence accelerated mice. J Toxicol Environ Health A. 2019;82: 1061–1068. DOI: https://doi.org/10.1080/15287394.2019.1683988
Grimm A, Biliouris EE, Lang UE, Götz J, Mensah-Nyagan AG, Eckert A. Sex hormone-related neurosteroids differentially rescue bioenergetic deficits induced by amyloid-β or hyperphosphorylated tau protein. Cell Mol Life Sci. 2016;73: 201–215. DOI: https://doi.org/10.1007/s00018-015-1988-x
Giannos P, Prokopidis K, Church DD, Kirk B, Morgan PT, Lochlainn MN, et al. Associations of Bioavailable Serum Testosterone With Cognitive Function in Older Men: Results From the National Health and Nutrition Examination Survey. Lipsitz LA, editor. J Gerontol Ser A. 2023;78: 151–157. DOI: https://doi.org/10.1093/gerona/glac162
Sundermann EE, Panizzon MS, Chen X, Andrews M, Galasko D, Banks SJ, et al. Sex differences in Alzheimer’s-related Tau biomarkers and a mediating effect of testosterone. Biol Sex Differ. 2020;11: 33. DOI: https://doi.org/10.1186/s13293-020-00310-x
Hogervorst E, Bandelow S, Combrinck M, Smith AD. Low free testosterone is an independent risk factor for Alzheimer’s disease. Exp Gerontol. 2004;39: 1633–1639. DOI: https://doi.org/10.1016/j.exger.2004.06.019
Rosario ER. Age-Related Testosterone Depletion and the Development of Alzheimer Disease. JAMA J Am Med Assoc. 2004;292: 1431–1432. DOI: https://doi.org/10.1001/jama.292.12.1431-b
Burkhardt MS, Foster JK, Clarnette RM, Chubb SAP, Bruce DG, Drummond PD, et al. Interaction between Testosterone and Apolipoprotein E ε4 Status on Cognition in Healthy Older Men. J Clin Endocrinol Metab. 2006;91: 1168–1172. DOI: https://doi.org/10.1210/jc.2005-1072
Tan S, Sohrabi HR, Weinborn M, Tegg M, Bucks RS, Taddei K, et al. Effects of Testosterone Supplementation on Separate Cognitive Domains in Cognitively Healthy Older Men: A Meta-analysis of Current Randomized Clinical Trials. Am J Geriatr Psychiatry. 2019;27: 1232–1246. DOI: https://doi.org/10.1016/j.jagp.2019.05.008
Huang G, Wharton W, Bhasin S, Harman SM, Pencina KM, Tsitouras P, et al. Effects of long-term testosterone administration on cognition in older men with low or low-to-normal testosterone concentrations: a prespecified secondary analysis of data from the randomised, double-blind, placebo-controlled TEAAM trial. Lancet Diabetes Endocrinol. 2016;4: 657–665. DOI: https://doi.org/10.1016/S2213-8587(16)30102-4
Mayes JS, Watson GH. Direct effects of sex steroid hormones on adipose tissues and obesity. Obes Rev. 2004;5: 197–216. DOI: https://doi.org/10.1111/j.1467-789X.2004.00152.x
Bornstein SR, Voit-Bak K, Rosenthal P, Tselmin S, Julius U, Schatz U, et al. Extracorporeal apheresis therapy for Alzheimer disease—targeting lipids, stress, and inflammation. Mol Psychiatry. 2020;25: 275–282. DOI: https://doi.org/10.1038/s41380-019-0542-x
Smith GD. Mendelian randomization: prospects, potentials, and limitations. Int J Epidemiol. 2004;33: 30–42. DOI: https://doi.org/10.1093/ije/dyh132
Skrivankova VW, Richmond RC, Woolf BAR, Davies NM, Swanson SA, VanderWeele TJ, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ. 2021; n2233. DOI: https://doi.org/10.1136/bmj.n2233
The Endometrial Cancer Association Consortium, Ruth KS, Day FR, Tyrrell J, Thompson DJ, Wood AR, et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med. 2020;26: 252–258. DOI: https://doi.org/10.1038/s41591-020-0751-5
Larsson SC, Woolf B, Gill D. Plasma Caffeine Levels and Risk of Alzheimer’s Disease and Parkinson’s Disease: Mendelian Randomization Study. Nutrients. 2022;14: 1697. DOI: https://doi.org/10.3390/nu14091697
Berndt SI, Gustafsson S, Mägi R, Ganna A, Wheeler E, Feitosa MF, et al. Genome-wide meta-analysis identifies 11 new loci for anthropometric traits and provides insights into genetic architecture. Nat Genet. 2013;45: 501–512. DOI: https://doi.org/10.1038/ng.2606
Burgess S, Thompson SG. Multivariable Mendelian Randomization: The Use of Pleiotropic Genetic Variants to Estimate Causal Effects. Am J Epidemiol. 2015;181: 251–260. DOI: https://doi.org/10.1093/aje/kwu283
Sanderson E, Smith GD, Windmeijer F, Bowden J. An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. Int J Epidemiol. 2018;0.
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife. 2018;7: e34408. DOI: https://doi.org/10.7554/eLife.34408
Hemani G, Bowden J, Davey Smith G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet. 2018;27: R195–R208. DOI: https://doi.org/10.1093/hmg/ddy163
Rees JMB, Wood AM, Burgess S. Extending the MR-Egger method for multivariable Mendelian randomization to correct for both measured and unmeasured pleiotropy. Stat Med. 2017;36: 4705–4718. DOI: https://doi.org/10.1002/sim.7492
Sanderson E, Spiller W, Bowden J. Testing and correcting for weak and pleiotropic instruments in two-sample multivariable Mendelian randomization. Stat Med. 2021;40: 5434–5452. DOI: https://doi.org/10.1002/sim.9133
Dong X, Jiang H, Li S, Zhang D. Low Serum Testosterone Concentrations Are Associated With Poor Cognitive Performance in Older Men but Not Women. Front Aging Neurosci. 2021;13: 712237. DOI: https://doi.org/10.3389/fnagi.2021.712237
Yang L, Zhou R, Tong Y, Chen P, Shen Y, Miao S, et al. Neuroprotection by dihydrotestosterone in LPS-induced neuroinflammation. Neurobiol Dis. 2020;140: 104814. DOI: https://doi.org/10.1016/j.nbd.2020.104814
Quaye E, Galecki AT, Tilton N, Whitney R, Briceño EM, Elkind MSV, et al. Association of Obesity With Cognitive Decline in Black and White Americans. Neurology. 2023;100: e220–e231. DOI: https://doi.org/10.1212/WNL.0000000000201367
Dake MD, De Marco M, Blackburn DJ, Wilkinson ID, Remes A, Liu Y, et al. Obesity and Brain Vulnerability in Normal and Abnormal Aging: A Multimodal MRI Study. J Alzheimers Dis Rep. 2021;5: 65–77. DOI: https://doi.org/10.3233/ADR-200267
Wingo AP, Vattathil SM, Liu J, Fan W, Cutler DJ, Levey AI, et al. LDL cholesterol is associated with higher AD neuropathology burden independent of APOE. J Neurol Neurosurg Psychiatry. 2022;93: 930–938. DOI: https://doi.org/10.1136/jnnp-2021-328164
Wingo TS, Cutler DJ, Wingo AP, Le N-A, Rabinovici GD, Miller BL, et al. Association of Early-Onset Alzheimer Disease With Elevated Low-Density Lipoprotein Cholesterol Levels and Rare Genetic Coding Variants of APOB. JAMA Neurol. 2019;76: 809. DOI: https://doi.org/10.1001/jamaneurol.2019.0648
Gagliano-Jucá T, Alvarez M, Basaria S. The medicalization of testosterone: reinventing the elixir of life. Rev Endocr Metab Disord. 2022;23: 1275–1284. DOI: https://doi.org/10.1007/s11154-022-09751-8
Wittert G, Grossmann M. Obesity, type 2 diabetes, and testosterone in ageing men. Rev Endocr Metab Disord. 2022;23: 1233–1242. DOI: https://doi.org/10.1007/s11154-022-09746-5
Mangolim AS, Brito LDAR, Nunes-Nogueira VDS. Effectiveness of testosterone replacement in men with obesity: a systematic review and meta-analysis. Eur J Endocrinol. 2022;186: 123–135. DOI: https://doi.org/10.1530/EJE-21-0473
Lapauw B, Kaufman J-M. MANAGEMENT OF ENDOCRINE DISEASE: Rationale and current evidence for testosterone therapy in the management of obesity and its complications. Eur J Endocrinol. 2020;183: R167–R183. DOI: https://doi.org/10.1530/EJE-20-0394
Zhang N, Zhang H, Zhang X, Zhang B, Wang F, Wang C, et al. The relationship between endogenous testosterone and lipid profile in middle-aged and elderly Chinese men. Eur J Endocrinol. 2014;170: 487–494. DOI: https://doi.org/10.1530/EJE-13-0802
Chuang Y-F, An Y, Bilgel M, Wong DF, Troncoso JC, O’Brien RJ, et al. Midlife adiposity predicts earlier onset of Alzheimer’s dementia, neuropathology and presymptomatic cerebral amyloid accumulation. Mol Psychiatry. 2016;21: 910–915. DOI: https://doi.org/10.1038/mp.2015.129
Matsuzaki T, Sasaki K, Hata J, Hirakawa Y, Fujimi K, Ninomiya T, et al. Association of Alzheimer disease pathology with abnormal lipid metabolism. Cerebrovasc Dis. 2011.
Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, et al. Adverse Events Associated with Testosterone Administration. N Engl J Med. 2010;363: 109–122. DOI: https://doi.org/10.1056/NEJMoa1000485
Sanderson E, Davey Smith G, Bowden J, Munafò MR. Mendelian randomisation analysis of the effect of educational attainment and cognitive ability on smoking behaviour. Nat Commun. 2019;10: 2949. DOI: https://doi.org/10.1038/s41467-019-10679-y
Carter AR, Sanderson E, Hammerton G, Richmond RC, Davey Smith G, Heron J, et al. Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Eur J Epidemiol. 2021;36: 465–478. DOI: https://doi.org/10.1007/s10654-021-00757-1
Acknowledgements
Not applicable.
Funding
Funding: None.
Author information
Authors and Affiliations
Contributions
Authors’ contributions: LLZ and LY initiated the study. LLZ undertook all statistical analysis. FY, JYM, and LY drafted the manuscript. XC, YXH, CW, and ML participated in the analysis. contributed to the interpretation of the analysis results and critical revision of the manuscript. LY managed the project. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate: All data used in this study were obtained from published GWAS studies. And all participants in each study provided informed consent, and all studies were approved by the relevant institutional review boards. Therefore, ethical approval was not required for this study because no unauthorized data at the individual level were used.
Consent for publication: Not applicable.
Competing interests: The authors declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhang, L., Yang, F., Ma, J. et al. The Impact of Testosterone on Alzheimer’s Disease Are Mediated by Lipid Metabolism and Obesity: A Mendelian Randomization Study. J Prev Alzheimers Dis 11, 507–513 (2024). https://doi.org/10.14283/jpad.2023.116
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jpad.2023.116