Abstract
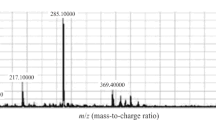
An LC-MS-MS method was revised and validated for simultaneous determination of icariin and its active metabolite icariside II in human plasma. The analytes and daidzein (IS) were extracted by liquid–liquid extraction and analyzed by LC-MS-MS. The separation was performed by a Zorbax SB-C18 column (3.5 μm, 2.1 × 100 mm) with an isocratic mobile phase consisting of methanol–water–formic acid (65:35:0.035, v/v/v) at a flow rate of 0.25 mL min−1. Detection was performed on a triple quadrupole tandem mass spectrum by multiple reaction monitoring mode using the electrospray ionization technique in positive mode. The method had lower limits of quantitation 0.2 and 0.1 ng mL−1 for icariin and icariside II, respectively, using 500 μL plasma sample. The linear calibration curves were obtained in the concentration range of 0.2–100 ng mL−1 for icariin and 0.1–100 ng mL−1 for icariside II. The RSD values of intra- and inter-day precision calculated from quality control (QC) samples were less than 7.2% for icariin and less than 6.5% for icariside II. The accuracy as determined from QC samples was within 3.8% for each analyte. The method has been applied to determine and evaluate the pharmacokinetic of icariin and its metabolite icariside II in volunteers following oral administration of icariin and extract of Epimedium, respectively.



Similar content being viewed by others
References
Editorial Committee of Pharmacopoeis of Ministry of Health PR China (2005) In: The Pharmacopoeia of People’s Republic of China (Part 1). China Chemical Industry Press, China
Chen KM, Ge BF, Ma HP, Liu XY (2005) Pharmazie 60:939–942
Huang X, Zhu D, Lou Y (2007) Eur J Pharmacol 564:26–36. doi:10.1016/j.ejphar.2007.02.039
Pan Y, Zhang WY, Xia X, Kong LD (2006) Biol Pharm Bull 29:2399–2403. doi:10.1248/bpb.29.2399
Zhang JW, Zhou YF, Wen XM, Wei L (2006) Clin J Exp Surg 23:1213–1214
Pan Y, Kong LD, Xia X, Zhang WY (2005) Pharmacol Biochem Behav 82:686–694. doi:10.1016/j.pbb.2005.11.010
Choi HJ, Ean JS, Kim DK, Li PH (2008) Eur J Pharma 579:58–65. doi:10.1016/j.ejphar.2007.10.010
Xu W, Zhang YP, Yang M, Shen ZY (2007) J Pharm Biomed Anal 45:667–672. doi:10.1016/j.jpba.2007.07.007
Acknowledgments
The work was supported by the program for Changjiang Scholars and Innovative Research Team in University (PCSIRT), NCET Foundation, NSFC(30725045), National 863 Program (2006AA02Z338), China Post-doctoral Science Foundation (20070410711), “973” program of China (2007CB507400), Shanghai Leading Academic Discipline Project (B906) and in part by the Scientific Foundation of Shanghai China (07DZ19728, 06DZ19717, 06DZ19005).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, YP., Xu, W., Li, N. et al. LC-MS-MS Method for Simultaneous Determination of Icariin and Its Active Metabolite Icariside II in Human Plasma. Chroma 68, 245–250 (2008). https://doi.org/10.1365/s10337-008-0685-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-008-0685-4