Abstract
Background
The effect of a single tumor marker on the prognosis of gastric cancer patients is not ideal. This study explored a novel prognostic assessment method for gastric cancer (GC) patients using a combination of three important tumor markers (CEA, CA72-4, and CA19-9).
Method
Data from 1966 GC patients who underwent curative gastrectomy at Sun Yat-Sen University Cancer Center (Guangzhou, China) were included. Hazard ratios (HR) for all factors for overall survival (OS) were analyzed by Cox regression. A nomogram and calibration curve were used to establish the survival prediction model. The prediction accuracy was evaluated with the concordance index (C-index).
Results
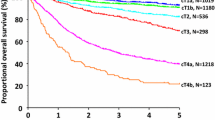
All patients were divided into four groups (C0–C3) according to the number of elevated tumor markers. The 5-year OS rates of the patients in preoperative groups C0–C3 were 83.8% (81.3–86.4%), 72.8% (68.5–77.4%), 58.9% (50.4–68.9%), and 18.5% (4.0–33.0%), respectively, and those in postoperative groups C0–C3 were 82.1% (79.4–84.8%), 76.1% (72.2–80.3%), 57.6% (48.4–68.5%), and 16.8% (5.1–28.5%), respectively, with significant differences between each C0–C3 subgroup in both preoperative and postoperative cohorts. Multivariate analysis showed that preoperative (HR: 6.001, 95% CI: 3.523–10.221) and postoperative (HR: 8.149, 95% CI: 4.962–13.528) elevated tumor markers were independent risk factors for GC patients. The C-index for the combined use of tumor markers was 0.65–0.66, which was higher than that for using a single tumor marker (0.53–0.56).
Conclusion
The combined use of tumor markers significantly improved the prognostic value compared with using a single tumor marker. The survival prediction model including the combined tumor markers was accurate and effective.




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Park SH, Sohn TS, Lee J, et al. Phase III Trial to Compare Adjuvant Chemotherapy With Capecitabine and Cisplatin Versus Concurrent Chemoradiotherapy in Gastric Cancer: Final Report of the Adjuvant Chemoradiotherapy in Stomach Tumors Trial, Including Survival and Subset Analyses. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 2015;33(28):3130–6.
Wang FH, Zhang XT, Li YF, et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Communications (London, England). 2021;41(8):747–95.
Edge S, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. New York: NY: Springer; 2010.
Huang C, Liu Z, Xiao L, et al. Clinical Significance of Serum CA125, CA19-9, CA72-4, and Fibrinogen-to-Lymphocyte Ratio in Gastric Cancer With Peritoneal Dissemination. Front Oncol. 2019;9:1159.
Bagaria B, Sood S, Sharma R, Lalwani S. Comparative study of CEA and CA19-9 in esophageal, gastric and colon cancers individually and in combination (ROC curve analysis). Cancer Biology & Medicine. 2013;10(3):148–57.
Shimada H, Noie T, Ohashi M, Oba K, Takahashi Y. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer. 2014;17(1):26–33.
Kim DH, Oh SJ, Oh CA, et al. The relationships between perioperative CEA, CA 19–9, and CA 72–4 and recurrence in gastric cancer patients after curative radical gastrectomy. Journal of Surgical Oncology. 2011;104(6):585–91.
Guo J, Chen S, Li S, et al. A novel classifier based on three preoperative tumor markers predicting the cancer-specific survival of gastric cancer (CEA, CA19-9 and CA72-4). Oncotarget. 2018;9(4):4814–22.
Hu PJ, Chen MY, Wu MS, et al. Clinical Evaluation of CA72-4 for Screening Gastric Cancer in a Healthy Population: A Multicenter Retrospective Study. Cancers. 2019;11(5).
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20(1):1–19. https://doi.org/10.1007/s10120-016-0622-4.
Sung JY, Cheong JH. Intercellular communications and metabolic reprogramming as new predictive markers for immunotherapy responses in gastric cancer. Cancer Communications (London, England). 2022;42(6):572–5.
Duffy MJ, Lamerz R, Haglund C, et al. Tumor markers in colorectal cancer, gastric cancer and gastrointestinal stromal cancers: European group on tumor markers 2014 guidelines update. Int J Cancer. 2014;134(11):2513–22.
Tong Y, Zhao Y, Shan Z, Zhang J. CA724 predicts overall survival in locally advanced gastric cancer patients with neoadjuvant chemotherapy. BMC Cancer. 2021;21(1):4.
Dilege E, Mihmanli M, Demir U, et al. Prognostic value of preoperative CEA and CA 19–9 levels in resectable gastric cancer. Hepato-gastroenterology. 2010;57(99–100):674–7.
Chen XZ, Zhang WK, Yang K, et al. Correlation between serum CA724 and gastric cancer: multiple analyses based on Chinese population. Molecular biology reports. 2012;39(9):9031–9.
Liu L, Xu H, Wang W, et al. A preoperative serum signature of CEA+/CA125+/CA19-9 ≥ 1000 U/mL indicates poor outcome to pancreatectomy for pancreatic cancer. International Journal of Cancer. 2015;136(9):2216–27.
Deng K, Yang L, Hu B, Wu H, Zhu H, Tang C. The prognostic significance of pretreatment serum CEA levels in gastric cancer: a meta-analysis including 14651 patients. PloS one. 2015;10(4):e0124151.
Rehena Z, Ghosh CK, Afroz F, et al. Comparison of Serum CA72-4 and CEA Levels in Patient with Endoscopically Suspected Gastric Carcinoma. Mymensingh Medical Journal: MMJ. 2015;24(3):542–9.
Feng F, Tian Y, Xu G, et al. Diagnostic and prognostic value of CEA, CA19-9, AFP and CA125 for early gastric cancer. BMC Cancer. 2017;17(1):737.
Wang W, Chen XL, Zhao SY, et al. Prognostic significance of preoperative serum CA125, CA19-9 and CEA in gastric carcinoma. Oncotarget. 2016;7(23):35423–36.
Lin JX, Wang W, Lin JP, et al. Preoperative Tumor Markers Independently Predict Survival in Stage III Gastric Cancer Patients: Should We Include Tumor Markers in AJCC Staging? Ann Surg Oncol. 2018;25(9):2703–12.
Wu T, Wang CH, Wang W, Liu LL, Yun JP, Zhou ZW. Association of preoperative and postoperative CA72-4 with gastric cancer outcome. J Surg Oncol. 2021;123(8):1699–707.
Uda H, Kanda M, Tanaka C, et al. Perioperative Serum Carcinoembryonic Antigen Levels Predict Recurrence and Survival of Patients with Pathological T2–4 Gastric Cancer Treated with Curative Gastrectomy. Digestive surgery. 2018;35(1):55–63.
Liang Y, Wang W, Fang C, et al. Clinical significance and diagnostic value of serum CEA, CA19-9 and CA72-4 in patients with gastric cancer. Oncotarget. 2016;7(31):49565–73.
Park SH, Ku KB, Chung HY, Yu W. Prognostic significance of serum and tissue carcinoembryonic antigen in patients with gastric adenocarcinomas. Cancer Research and Treatment. 2008;40(1):16–21.
Nakamura Y, Shida D, Tanabe T, et al. Prognostic impact of preoperatively elevated and postoperatively normalized carcinoembryonic antigen levels following curative resection of stage I-III rectal cancer. Cancer Med. 2020;9(2):653–62.
Konishi T, Shimada Y, Hsu M, et al. Association of Preoperative and Postoperative Serum Carcinoembryonic Antigen and Colon Cancer Outcome. JAMA Oncol. 2018;4(3):309–15.
Snyder RA, Hu CY, Cuddy A, et al. Association Between Intensity of Posttreatment Surveillance Testing and Detection of Recurrence in Patients With Colorectal Cancer. JAMA. 2018;319(20):2104–15.
Chen S, Chen YB, Li YF, et al. Normal carcinoembryonic antigen indicates benefit from perioperative chemotherapy to gastric carcinoma patients. World Journal of Gastroenterology. 2012;18(29):3910–6.
Lee SE, Lee JH, Ryu KW, et al. Preoperative plasma fibrinogen level is a useful predictor of adjacent organ involvement in patients with advanced gastric cancer. Journal of Gastric Cancer. 2012;12(2):81–7.
Wang Y, Zhao C, Chang L, et al. Circulating tumor DNA analyses predict progressive disease and indicate trastuzumab-resistant mechanism in advanced gastric cancer. EBioMedicine. 2019;43:261–9.
Reddavid R, Sofia S, Chiaro P, Colli F, Trapani R, Esposito L, Solej M, Degiuli M. Neoadjuvant chemotherapy for gastric cancer. Is it a must or a fake? World J Gastroenterol. 2018 Jan 14;24(2):274-289.
Li S, Yu W, Xie F, Luo H, Liu Z, Lv W, Shi D, Yu D, Gao P, Chen C, Wei M, Zhou W, Wang J, Zhao Z, Dai X, Xu Q, Zhang X, Huang M, Huang K, Wang J, Li J, Sheng L, Liu L. Neoadjuvant therapy with immune checkpoint blockade, anti-angiogenesis, and chemotherapy for locally advanced gastric cancer. Nat Commun. 2023;14(1):8.
Rausei S, Bali CD, Lianos GD. Neoadjuvant chemotherapy for gastric cancer. Has the time to decelerate the enthusiasm passed us by? Semin Oncol. 2020 Dec;47(6):355-360.
Ajani JA, D'Amico TA, Bentrem DJ, et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. JNCCN. 2022; 20(2): 167-92.
Acknowledgment
We thank all staff of the Department of Gastric Surgery at SYSUCC for assisting in data collection for this study.
Funding
This work was supported by grants from the National Science Foundation of China (82103586), Science and Technology Planning Project of Guangzhou (202201010885), Guangdong Provincial Natural Science Foundation (2021A1515012369), and Beijing Xisike Clinical Oncology Research Foundation (Y-tongshu2021/qn-0227).
Author information
Authors and Affiliations
Contributions
Y.B.C. and R.C.N. conceptualized the study and devised the study protocol. R.P.Z., X.J.C., and G.M.C. participated in the study design and manuscript writing. Z.K.Z. and Y.C.W. participated in data collection. F.Y.Z. and J.L. developed the methodology and statistical analysis. All authors approved the final version of the manuscript. Y.B.C. and R.C.N. have full access to, and verify, the underlying data and accept responsibility for submission and publication.
Corresponding authors
Ethics declarations
Disclosure
The authors declare no competing interests.
Consent for Publication
All authors gave their consent for publication.
Ethical Approval and Consent to Participate
This study was approved by the Institutional Review Board and Human Ethics Committee of SYSUCC. Written consent to use the samples for research purposes was obtained from all the patients before surgery. The project was examined by the Animal Ethical and Welfare Committee of SYSUCC and complies with animal protection, animal welfare, ethical principles, and the relevant provisions of the National Laboratory Animal Welfare Ethics.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, R., Chen, X., Chen, G. et al. Combined Use of Tumor Markers in Gastric Cancer: A Novel Method with Promising Prognostic Accuracy and Practicality. Ann Surg Oncol 30, 8561–8571 (2023). https://doi.org/10.1245/s10434-023-14194-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-14194-9