Abstract
Purpose
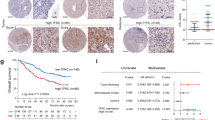
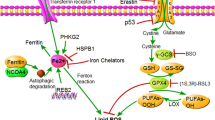
Transferrin receptor (TFR), a membrane protein that has a critical role in the transport of iron into cells, is known to be a ferroptosis-related marker. Although TFR is reported to be abundantly expressed in tumor cells, its relationship with ferroptosis inducers in hepatocellular carcinoma (HCC) remains unclear.
Methods
The authors performed immunohistochemical staining of TFR and divided 350 HCC patients into two groups according to its expression. They analyzed the association between TFR expression and prognosis or clinicopathologic factors. In addition, the regulation of malignant activity and its effect on the efficacy of ferroptosis inducers were investigated in vitro.
Results
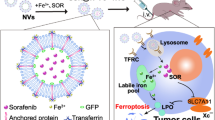
For this study, 350 patients were divided into TFR-positive (n =180, 51.4%) and TFR-negative (n = 170, 48.6%) groups. The TFR-positive group had more hepatitis B surface antigen (HBs-Ag) (p = 0.0230), higher α-fetoprotein (AFP) levels (p = 0.0023), higher des-gamma-carboxyprothrombin (DCP) levels (p = 0.0327), a larger tumor size (p = 0.0090), greater proportions of Barcelona Clinic Liver Cancer (BCLC) stage B or C (p = 0.0005), poor differentiation (p < 0.0001), and microscopic intrahepatic metastasis (p = 0.0066). In the multivariate analyses, TFR expression was an independent prognostic factor in disease-free survival (p = 0.0315). In vitro, TFRC knockdown decreased cell motility. In addition, TFRC knockdown abolished artesunate (AS)-, lenvatinib-, and sorafenib-induced ferroptosis in HCC cell lines. The study demonstrated that simultaneous treatment of AS with multi-kinase inhibitor augmented the ferroptosis-inducing effects of AS in HCC cell lines.
Conclusion
TFR expression is a poor prognostic factor in HCC, but its expression increases sensitivity to ferroptosis-inducing agents.







Similar content being viewed by others
References
Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: new trends. J Hepatol. 2020;72:250–61. https://doi.org/10.1016/j.jhep.2019.08.025.
Okamura Y, Sugiura T, Ito T, et al. Changes in patient background and prognosis after hepatectomy for hepatocellular carcinoma by hepatitis virus infection status: new trends in Japan. Ann Gastroenterological Surg. 2021;5:553–66. https://doi.org/10.1002/ags3.12451.
Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a envatinib phase 3 non-inferiority trial. Lancet. 2018;391:1163–73. https://doi.org/10.1016/s0140-6736(18)30207-1.
Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from Imbrave150: atezolizumab plus bevacizumab vs sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862–73. https://doi.org/10.1016/j.jhep.2021.11.030.
Itoh S, Yoshizumi T, Yugawa K, et al. Impact of immune response on outcomes in hepatocellular carcinoma: association with vascular formation. Hepatology. 2020;72:1987–99. https://doi.org/10.1002/hep.31206.
Itoh S, Yoshizumi T, Kitamura Y, et al. Impact of metabolic activity in hepatocellular carcinoma: association with immune status and vascular formation. Hepatology Commun. 2021;5:1278–89. https://doi.org/10.1002/hep4.1715.
Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. https://doi.org/10.1016/j.cell.2012.03.042.
Lei G, Zhuang L, Gan B. Targeting ferroptosis as a vulnerability in cancer. Nat Rev Cancer. 2022;22:381–96. https://doi.org/10.1038/s41568-022-00459-0.
Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell. 2015;59:298–308. https://doi.org/10.1016/j.molcel.2015.06.011.
Adachi M, Kai K, Yamaji K, et al. Transferrin receptor 1 overexpression is associated with tumour de-differentiation and acts as a potential prognostic indicator of hepatocellular carcinoma. Histopathology. 2019;75:63–73. https://doi.org/10.1111/his.13847.
Li ZJ, Dai HQ, Huang XW, et al. Artesunate synergizes with sorafenib to induce ferroptosis in hepatocellular carcinoma. Acta Pharmacol Sin. 2021;42:301–10. https://doi.org/10.1038/s41401-020-0478-3.
Farshidfar F, Zheng S, Gingras MC, et al. Integrative genomic analysis of cholangiocarcinoma identifies distinct IDH-mutant molecular profiles. Cell Rep. 2017;18:2780–94. https://doi.org/10.1016/j.celrep.2017.02.033.
Vasaikar SV, Straub P, Wang J, Zhang B. LinkedOmics: analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res. 2017. https://doi.org/10.1093/nar/gkx1090.
Itoh S, Tomiyama T, Morinaga A, et al. Clinical effects of the use of the indocyanine green fluorescence imaging technique in laparoscopic partial liver resection. Ann Gastroenterol Surg. 2022;6:688–94. https://doi.org/10.1002/ags3.12563.
Toshida K, Itoh S, Harada N, et al. Cancer-associated fibroblasts promote tumor cell growth via miR-493-5p in intrahepatic cholangiocarcinoma. Cancer Sci. 2023;114:937–47. https://doi.org/10.1111/cas.15644.
Itoh S, Taketomi A, Harimoto N, et al. Antineoplastic effects of gamma linolenic acid on hepatocellular carcinoma cell lines. J Clin Biochem Nutr. 2010;47:81–90. https://doi.org/10.3164/jcbn.10-24.
Lin W, Tsai W, Shao R, et al. Hepatitis C virus regulates transforming growth factor β1 production through the generation of reactive oxygen species in a nuclear factor κB-dependent manner. Gastroenterology. 2010;138:2509-2518.e1. https://doi.org/10.1053/j.gastro.2010.03.008.
Yang X, Zheng Y, Liu L, Huang J, Wang F, Zhang J. Progress on the study of the anticancer effects of artesunate. Oncol Lett. 2021;22:750. https://doi.org/10.3892/ol.2021.13011.
Kawabata H. Transferrin and transferrin receptors update. Free Radical Bio Med. 2019;133:46–54. https://doi.org/10.1016/j.freeradbiomed.2018.06.037.
Shen Y, Li X, Dong D, Zhang B, Xue Y, Shang P. Transferrin receptor 1 in cancer: a new sight for cancer therapy. Am J Cancer Res. 2018;8:916–31.
Shen Y, Li X, Zhao B, et al. Iron metabolism gene expression and prognostic features of hepatocellular carcinoma. J Cell Biochem. 2018;119:9178–204. https://doi.org/10.1002/jcb.27184.
Sun H, Qian X, Yang W, et al. Novel prognostic signature based on HRAS, MAPK3, and TFRC identified to be associated with ferroptosis and the immune microenvironment in hepatocellular carcinoma. Am J Transl Res. 2022;14:6924–40.
Asare GA, Mossanda KS, Kew MC, Paterson AC, Kahler-Venter CP, Siziba K. Hepatocellular carcinoma caused by iron overload: a possible mechanism of direct hepatocarcinogenicity. Toxicology. 2006;219:41–52. https://doi.org/10.1016/j.tox.2005.11.006.
Sorrentino P, D’Angelo S, Ferbo U, Micheli P, Bracigliano A, Vecchione R. Liver iron excess in patients with hepatocellular carcinoma developed on non-alcoholic steato-hepatitis. J Hepatol. 2009;50:351–7. https://doi.org/10.1016/j.jhep.2008.09.011.
Malumbres M, Barbacid M. Cell cycle, CDKs, and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–66. https://doi.org/10.1038/nrc2602.
Li J, Cao F, Yin HL, et al. Ferroptosis: past, present, and future. Cell Death Dis. 2020;11:88. https://doi.org/10.1038/s41419-020-2298-2.
Sun X, Ou Z, Chen R, et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63:173–84. https://doi.org/10.1002/hep.28251.
Yao F, Deng Y, Zhao Y, et al. A targetable LIFR−NF-κB−LCN2 axis controls liver tumorigenesis and vulnerability to ferroptosis. Nat Commun. 2021;12:7333. https://doi.org/10.1038/s41467-021-27452-9.
Bekric D, Ocker M, Mayr C, et al. Ferroptosis in hepatocellular carcinoma: mechanisms, drug targets, and approaches to clinical translation. Cancers. 2022;14:1826. https://doi.org/10.3390/cancers14071826.
Hino K, Yanatori I, Hara Y, Nishina S. Iron and liver cancer: an inseparable connection. Febs J. 2022;289:7810–29. https://doi.org/10.1111/febs.16208.
Iseda N, Itoh S, Toshida K, et al. Ferroptosis is induced by lenvatinib through fibroblast growth factor receptor-4 inhibition in hepatocellular carcinoma. Cancer Sci. 2022;113:2272–87. https://doi.org/10.1111/cas.15378.
Dixon SJ, Patel DN, Welsch M, et al. Pharmacological inhibition of cystine–glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife. 2014;3:e02523. https://doi.org/10.7554/elife.02523.
Acknowledgements
This study was supported by JSPS KAKENHI (Grant No. JP-19K09198) and by the Takeda Science Foundation. The funding sources had no role in the collection, analysis, or interpretation of the data or in the decision to submit the article for publication. We thank Ms. Saori Tsurumaru, Ms. Asuka Nakamura, Ms. Yuko Kubota, and Ms. Miki Nakashima for technical support. We also thank H. Nikki March, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this report.
Author information
Authors and Affiliations
Contributions
MH and KT participated in the study conception and design, analysis, and drafting of the article. SI participated in the study conception and design, and in the critical revision of the manuscript. NH and KK participated in the data acquisition, analysis, and interpretation. YO and TY participated in the analysis participated in the critical revision of the manuscript.
Corresponding author
Ethics declarations
Disclosure
The authors declare that they have no conflict of interest.
Informed Consent
All patients provided informed consent. Registry and the Registration No. of the study: This retrospective study was approved by the ethics committee of Kyushu University (approval code: 2021-467).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10434_2023_14053_MOESM1_ESM.tiff
Fig. S1 Gene set enrichment analysis (GSEA) shows that TFRC is highly related to the cell cycle. A Normal enrichment score (NES). B Cell cycle-related enrichment plots (TIFF 33973 KB)
10434_2023_14053_MOESM2_ESM.tiff
Fig. S2 Relationship between intracellular iron levels and efficacy of ferroptosis inducers. A Intracellular iron levels in DFO-treated cells 48 h after treatment. B Intracellular iron levels in TFRC knockdown (KD) cells 48 h after gene-silencing. C Cell proliferation ability was reduced in iron-chelated cells. Data are presented as median ± range. *p < 0.05. **p < 0.001. ***p < 0.0001. DFO, deferoxamine mesylate (TIFF 33973 KB)
10434_2023_14053_MOESM3_ESM.tiff
Fig. S3 Combination treatment augments the efficacy of sorafenib (SOR) multi-kinase inhibitor. Huh7 and PLC cells were treated with artesunate (AS) (25 µmol) and/or SOR (40 µmol). Cell viability, ROS, and MDA levels were measured 72 h after cell seeding. Combination treatment with lenvatinib (LEN) and AS resulted in (A) reduced cell viability, (B) increased ROS, and (C) MDA accumulation, indicating increased sensitivity to ferroptosis compared with SOR treatment alone. Data are presented as median ± range. *p < 0.05. **p < 0.001. ***p < 0.0001. MDA, malondialdehyde (TIFF 33973 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hiromatsu, M., Toshida, K., Itoh, S. et al. Transferrin Receptor is Associated with Sensitivity to Ferroptosis Inducers in Hepatocellular Carcinoma. Ann Surg Oncol 30, 8675–8689 (2023). https://doi.org/10.1245/s10434-023-14053-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-14053-7