Abstract
Introduction
The study investigated the synergistic effect of the micropapillary (MIP) component and consolidation-to-tumor ratio (CTR) on the recurrence and survival of patients with pathologic stage IA3 lung adenocarcinoma.
Methods
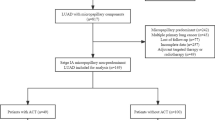
We enrolled 419 patients confirmed pathological stage IA3 adenocarcinoma from four institutions. Kaplan–Meier analysis was performed to examine the value of the MIP component and CTR on relapse-free survival (RFS) and overall survival (OS). The cumulative recurrence between different stages was analyzed by using cumulative event curves.
Results
RFS (P < 0.0001) and OS (P = 0.008) in the presence of the MIP group were significantly lower than those in the absence of the MIP group, and CTR > 5 only reduced RFS (P = 0.0004), but not OS (P = 0.063), in the patients. In addition, the prognosis of patients with both the MIP component and CTR > 5 was worse than that of those without the MIP component or CTR ≤ 5. Therefore, we established new subtypes of the stage IA3: IA3a, IA3b, and IA3c. RFS and OS for IA3c staging were significantly lower than those for IA3a and IA3b. For IA3c, the cumulative incidence of local recurrence (P < 0.001) and that of distant metastasis (P = 0.004) were significantly higher than those for IA3a and IA3b.
Conclusions
The MIP component combined with CTR > 0.5 can effectively predict the prognosis of patients with pathological stage IA3 lung adenocarcinoma and may offer more detailed recurrence and survival information according to the established subtype stage of IA3.





Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. Lancet. 2012;398:535–54.
Demicheli R, Fornili M, Ambrogi F, Higgins K, Boyd J, Biganzoli E, Kelsey C. Recurrence dynamics for non-small-cell lung cancer: effect of surgery on the development of metastases. J Thorac Oncol. 2012;7:723–30.
Yun JK, Lee GD, Choi S, Kim YH, Kim DK, Park SI, Kim HR. Various recurrence dynamics for non-small cell lung cancer depending on pathological stage and histology after surgical resection. Transl Lung Cancer Res. 2022;11:1327–36.
Kelsey CR, Fornili M, Ambrogi F, Higgins K, Boyd JA, Biganzoli E, Demicheli R. Metastasis dynamics for non-small-cell lung cancer: effect of patient and tumor-related factors. Clin Lung Cancer. 2013;14:425–32.
Miller M. Hanna N (2021) Advances in systemic therapy for non-small cell lung cancer. BMJ Clin Res. 2021;375:n2363.
Ko E, Raben D, Formenti S. The integration of radiotherapy with immunotherapy for the treatment of non-small cell lung cancer. Clin Cancer Res. 2018;24:5792–806.
Hattori A, Matsunaga T, Takamochi K, Oh S, Suzuki K. Prognostic impact of a ground glass opacity component in the clinical T classification of non-small cell lung cancer. J Thorac Cardiovasc Surg. 2017;154:2102-2110.e2101.
Ye T, Deng L, Wang S, Xiang J, Zhang Y, Hu H, Sun Y, Li Y, Shen L, Xie L, Gu W, Zhao Y, Fu F, Peng W, Chen H. Lung adenocarcinomas manifesting as radiological part-solid nodules define a special clinical subtype. J Thorac Oncol. 2019;14:617–27.
Shigefuku S, Shimada Y, Hagiwara M, Kakihana M, Kajiwara N, Ohira T, Ikeda N. Prognostic significance of ground-glass opacity components in 5-year survivors with resected lung adenocarcinoma. Ann Surg Oncol. 2021;28:148–56.
Fu F, Zhang Y, Wen Z, Zheng D, Gao Z, Han H, Deng L, Wang S, Liu Q, Li Y, Shen L, Shen X, Zhao Y, Zhao Z, Ye T, Xiang J, Zhang Y, Sun Y, Hu H, Chen H. Distinct prognostic factors in patients with stage i non-small cell lung cancer with radiologic part-solid or solid lesions. J Thorac Oncol. 2019;14:2133–42.
Hattori A, Matsunaga T, Takamochi K, Oh S, Suzuki K. Neither maximum tumor size nor solid component size is prognostic in part-solid lung cancer: Impact of tumor size should be applied exclusively to solid lung cancer. Ann Thorac Surg. 2016;102:407–15.
Zhang S, Lin D, Yu Y, Cao Q, Liu G, Jiang D, Wang H, Fang Y, Shen Y, Yin J, Hou Y, Shi H, Ge D, Wang Q, Tan L. Which will carry more weight when CTR > 0.5, solid component size, CTR, tumor size or SUVmax? Lung Cancer. 2022;164:14–22.
Ito H, Suzuki K, Mizutani T, Aokage K, Wakabayashi M, Fukuda H, Watanabe SI. Long-term survival outcome after lobectomy in patients with clinical T1 no lung cancer. J Thorac Cardiovasc Surg. 2020. https://doi.org/10.1016/j.jtcvs.2019.12.072.
Kamiya K, Hayashi Y, Douguchi J, Hashiguchi A, Yamada T, Izumi Y, Watanabe M, Kawamura M, Horinouchi H, Shimada N, Kobayashi K, Sakamoto M. Histopathological features and prognostic significance of the micropapillary pattern in lung adenocarcinoma. Mod Pathol. 2008;21:992–1001.
Zhao Y, Wang R, Shen X, Pan Y, Cheng C, Li Y, Shen L, Zhang Y, Li H, Zheng D, Ye T, Zheng S, Sun Y, Chen H. Minor components of micropapillary and solid subtypes in lung adenocarcinoma are predictors of lymph node metastasis and poor prognosis. Ann Surg Oncol. 2016;23:2099–105.
Watanabe K, Sakamaki K, Ito H, Yokose T, Yamada K, Nakayama H, Masuda M. Impact of the micropapillary component on the timing of recurrence in patients with resected lung adenocarcinoma. Eur J Cardiothorac Surg. 2020;58:1010–8.
Wood DE, Kazerooni EA, Baum SL, Eapen GA, Ettinger DS, Hou L, Jackman DM, Klippenstein D, Kumar R, Lackner RP, Leard LE, Lennes IT, Leung ANC, Makani SS, Massion PP, Mazzone P, Merritt RE, Meyers BF, Midthun DE, Pipavath S, Pratt C, Reddy C, Reid ME, Rotter AJ, Sachs PB, Schabath MB, Schiebler ML, Tong BC, Travis WD, Wei B, Yang SC, Gregory KM, Hughes M. Lung cancer screening, version 3. 2018, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2018;16:412–41.
Dai C, Xie H, Kadeer X, Su H, Xie D, Ren Y, She Y, Zhu E, Fan Z, Chen T, Qin L, Zheng H, Zhang L, Jiang G, Wu C, Chen C. Relationship of lymph node micrometastasis and micropapillary component and their joint influence on prognosis of patients with stage I lung adenocarcinoma. Am J Surg Pathol. 2017;41:1212–20.
Hung JJ, Jeng WJ, Chou TY, Hsu WH, Wu KJ, Huang BS, Wu YC. Prognostic value of the new international association for the study of lung cancer/American thoracic society/European respiratory society lung adenocarcinoma classification on death and recurrence in completely resected stage I lung adenocarcinoma. Ann Surg. 2013;258:1079–86.
Morimoto J, Nakajima T, Suzuki H, Nagato K, Iwata T, Yoshida S, Fukuyo M, Ota S, Nakatani Y, Yoshino I. Impact of free tumor clusters on prognosis after resection of pulmonary adenocarcinoma. J Thorac Cardiovasc Surg. 2016;152:64-72.e61.
Asamura H, Chansky K, Crowley J, Goldstraw P, Rusch VW, Vansteenkiste JF, Watanabe H, Wu YL, Zielinski M, Ball D, Rami-Porta R. The international association for the study of lung cancer lung cancer staging project: proposals for the revision of the N descriptors in the forthcoming 8th edition of the TNM classification for lung cancer. J Thorac Oncol. 2015;10:1675–84.
Suzuki K, Watanabe SI, Wakabayashi M, Saji H, Aokage K, Moriya Y, Yoshino I, Tsuboi M, Nakamura S, Nakamura K, Mitsudomi T, Asamura H. A single-arm study of sublobar resection for ground-glass opacity dominant peripheral lung cancer. J Thorac Cardiovasc Surg. 2022;163:289-301.e282.
Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K, Aoki T, Okami J, Yoshino I, Ito H, Okumura N, Yamaguchi M, Ikeda N, Wakabayashi M, Nakamura K, Fukuda H, Nakamura S, Mitsudomi T, Watanabe SI, Asamura H. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet. 2022;399:1607–17.
Matsunaga T, Suzuki K, Takamochi K, Oh S. What is the radiological definition of part-solid tumour in lung cancer?†. Eur J Cardiothorac Surg. 2017;51:242–7.
Huang W, Zhang H, Zhang Z, Zhang B, Sun X, Huo Y, Feng Y, Tian P, Mo H, Wang C. A prognostic nomogram based on a new classification of combined micropapillary and solid components for stage IA invasive lung adenocarcinoma. J Surg Oncol. 2022;125:796–808.
Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P, Mitchell A, Bolejack V. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39–51.
Watanabe K, Tsuboi M, Sakamaki K, Nishii T, Yamamoto T, Nagashima T, Ando K, Ishikawa Y, Woo T, Adachi H, Kumakiri Y, Maehara T, Nakayama H, Masuda M. Postoperative follow-up strategy based on recurrence dynamics for non-small-cell lung cancer. Eur J Cardiothorac Surg. 2016;49:1624–31.
Funding
This study was supported by grants from the Key Laboratory of Cardio-Thoracic Surgery (Fujian Medical University), Fujian Province University (No.0713304); the Natural Science Foundation in Fujian Province (No. 2020J011004); the Fujian provincial health technology project (No. 2020CXA028); the cohort study of the School of Public Health, Fujian Medical University (No. 2021HX003); the Joint Funds for the innovation of science and Technology, Fujian province (No.2020Y9076); and the National Nature Science Foundation of China (No.82273415).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
The authors declare no potential conflicts of interest.
Ethical Approval
This study was reviewed and approved by the Institutional Ethics Review Committee of the Fujian Medical University Union Hospital (IRB: No. 2018KY033)
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, Sj., Tu, Jh., Chen, H. et al. A Multi-institutional Analysis of the Combined Effect of Micropapillary Component and Consolidation-to-Tumor Ratio >0.5 on the Prognosis of Pathological, Stage IA3, Lung Adenocarcinoma. Ann Surg Oncol 30, 5843–5853 (2023). https://doi.org/10.1245/s10434-023-13658-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-13658-2