Abstract
Background
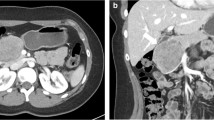
Solid pseudopapillary neoplasms (SPN) are rare tumors of the pancreas, typically affecting young women. Resection is the mainstay of treatment but is associated with significant morbidity and potential mortality. We explore the idea that small, localized SPN could be safely observed.
Methods
This retrospective review of the Pancreas National Cancer Database from 2004 to 2018 identified SPN via histology code 8452.
Results
A total of 994 SPNs were identified. Mean age was 36.8 ± 0.5 years, 84.9% (n = 844) were female, and most had a Charlson–Deyo Comorbidity Coefficient (CDCC) of 0–1 (96.6%, n = 960). Patients were most often staged clinically as cT2 (69.5%, n = 457) followed by cT3 (17.6%, n = 116), cT1 (11.2%, n = 74), and cT4 (1.7%, n = 11). Clinical lymph node and distant metastasis rates were 3.0 and 4.0%, respectively. Surgical resection was performed in 96.6% of patients (n = 960), most commonly partial pancreatectomy (44.3%) followed by pancreatoduodenectomy (31.3%) and total pancreatectomy (8.1%). In patients clinically staged as node (N0) and distant metastasis (M0) negative, occult pathologic lymph node involvement was found in 0% (n = 28) of patients with stage cT1 and 0.5% (n = 185) of patients with cT2 disease. The risk of occult nodal metastasis significantly increased to 8.9% (n = 61) for patients with cT3 disease. The risk further increased to 50% (n = 2) in patients with cT4 disease.
Conclusions
Herein, the specificity of excluding nodal involvement clinically is 99.5% in tumors ≤ 4 cm and 100% in tumors ≤ 2 cm. Therefore, there may be a role for close observation in patients with cT1N0 lesions to mitigate morbidity from major pancreatic resection.


Similar content being viewed by others
References
Torres OJM, Rezende MB, Waechter FL, et al. Pancreatoduodenectomy for solid pseudopapillary tumor of the pancreas: a multi-institution study. Arq Bras Cir Dig. 2019;32(2):e1442.
Hamilton S, Aaltonen L, World Health Organization classificaiton of tumors. Pathology and genetics of tumours of the digestive system. USA: IARC Press; 2000.
Vollmer CM Jr, Dixon E, Grant DR. Management of a solid pseudopapillary tumor of the pancreas with liver metastases. HPB. 2003;5(4):264–7.
Yagci A, Yakan S, Coskun A, et al. Diagnosis and treatment of solid pseudopapillary tumor of the pancreas: experience of one single institution from Turkey. World J Surg Oncol. 2013;11:308.
Lanke G, Ali FS, Lee JH. Clinical update on the management of pseudopapillary tumor of pancreas. World J Gastrointest Endosc. 2018;10(9):145–55.
Law JK, Ahmed A, Singh VK, et al. A systematic review of solid-pseudopapillary neoplasms: are these rare lesions? Pancreas. 2014;43(3):331–7.
Romics L Jr, Olah A, Belagyi T, Hajdu N, Gyurus P, Ruszinko V. Solid pseudopapillary neoplasm of the pancreas–proposed algorithms for diagnosis and surgical treatment. Langenbecks Arch Surg. 2010;395(6):747–55.
Shuja A, Alkimawi KA. Solid pseudopapillary tumor: a rare neoplasm of the pancreas. Gastroenterol Rep. 2014;2(2):145–9.
Abudalou M, Vega EA, Dhingra R, et al. Solid pseudopapillary neoplasm-diagnostic approach and post-surgical follow up: three case reports and review of literature. World J Clin Cases. 2021;9(7):1682–95.
Jena SS, Ray S, Das SAP, Mehta NN, Yadav A, Nundy S. Rare Pseudopapillary neoplasm of the pancreas: a 10-year experience. Surg Res Pract. 2021;2021:7377991.
Lubezky N, Papoulas M, Lessing Y, et al. Solid pseudopapillary neoplasm of the pancreas: management and long-term outcome. Eur J Surg Oncol. 2017;43(6):1056–60.
Martin RC, Klimstra DS, Brennan MF, Conlon KC. Solid-pseudopapillary tumor of the pancreas: a surgical enigma? Ann Surg Oncol. 2002;9(1):35–40.
Sharma I, Cho WC, Thaker S, et al. Solid pseudopapillary neoplasm of the pancreas. J Pediatr Surg Case Rep. 2018;37:63–9.
Stensbol AB, Krogh J, Holmager P, et al. Incidence, Clinical presentation and trends in indication for diagnostic work-up of small intestinal and pancreatic neuroendocrine tumors. Diagnostics. 2021;11(11):2030.
Santo E, Bar-Yishay I. Pancreatic solid incidentalomas. Endosc Ultrasound. 2017;6(Suppl 3):S99–103.
Yu PF, Hu ZH, Wang XB, et al. Solid pseudopapillary tumor of the pancreas: a review of 553 cases in Chinese literature. World J Gastroenterol. 2010;16(10):1209–14.
De Robertis R, Marchegiani G, Catania M, et al. Solid pseudopapillary neoplasms of the pancreas: clinicopathologic and radiologic features according to size. AJR Am J Roentgenol. 2019;213(5):1073–80.
Albuquerque GPX, Ramos A, Anaissi AKM, et al. Hepatic metastasis in Frantz’s tumor: a case report. Int J Surg Case Rep. 2020;71:66–9.
Distler M, Ruckert F, Hunger M, et al. Evaluation of survival in patients after pancreatic head resection for ductal adenocarcinoma. BMC Surg. 2013;13:12.
Cusworth BM, Krasnick BA, Nywening TM, et al. Whipple-specific complications result in prolonged length of stay not accounted for in ACS-NSQIP Surgical Risk Calculator. HPB. 2017;19(2):147–53.
Pugalenthi A, Protic M, Gonen M, et al. Postoperative complications and overall survival after pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. J Surg Oncol. 2016;113(2):188–93.
Zhang C, Liu F, Chang H, et al. Less aggressive surgical procedure for treatment of solid pseudopapillary tumor: limited experience from a single institute. PLoS ONE. 2015;10(11):e0143452.
Wu L, Nahm CB, Jamieson NB, et al. Risk factors for development of diabetes mellitus (Type 3c) after partial pancreatectomy: a systematic review. Clin Endocrinol. 2020;92(5):396–406.
Burkhart RA, Gerber SM, Tholey RM, et al. Incidence and severity of pancreatogenic diabetes after pancreatic resection. J Gastrointest Surg. 2015;19(2):217–25.
Lee LC, Grant CS, Salomao DR, et al. Small, nonfunctioning, asymptomatic pancreatic neuroendocrine tumors (PNETs): role for nonoperative management. Surgery. 2012;152(6):965–74.
Gaujoux S, Partelli S, Maire F, et al. Observational study of natural history of small sporadic nonfunctioning pancreatic neuroendocrine tumors. J Clin Endocrinol Metab. 2013;98(12):4784–9.
Bettini R, Partelli S, Boninsegna L, et al. Tumor size correlates with malignancy in nonfunctioning pancreatic endocrine tumor. Surgery. 2011;150(1):75–82.
Gallotti A, Johnston RP, Bonaffini PA, et al. Incidental neuroendocrine tumors of the pancreas: MDCT findings and features of malignancy. AJR Am J Roentgenol. 2013;200(2):355–62.
Yoon HJ, Lim JH. Solid pseudopapillary tumor of the pancreas with hepatic metastasis: spontaneous regression over 10-year follow-up period. Korean J Radiol. 2012;13(5):648–51.
Hachiya M, Hachiya Y, Mitsui K, Tsukimoto I, Watanabe K, Fujisawa T. Solid, cystic and vanishing tumors of the pancreas. Clin Imaging. 2003;27(2):106–8.
Suzuki M, Shimizu T, Minowa K, Ikuse T, Baba Y, Ohtsuka Y. Spontaneous shrinkage of a solid pseudopapillary tumor of the pancreas: CT findings. Pediatr Int. 2010;52(2):335–6.
Tanaka M, Fernandez-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12(3):183–97.
Vege SS, Ziring B, Jain R, Moayyedi P, Clinical Guidelines C, American Gastroenterology A. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148(4):819–22.
(NCCN) NCCN. NCCN Clinical Practice Guidelines in Oncology. Neuroendocrine Adrenal Tumor (Version 1.2022). 2022.
Partelli S, Ramage JK, Massironi S, et al. Management of asymptomatic sporadic nonfunctioning pancreatic neuroendocrine neoplasms (ASPEN) ≤ 2 cm: study protocol for a prospective observational study. Front Med. 2020;7:598438.
Funding
There was no outside funding or influence in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
There are no conflicts of interest for any of the authors listed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Standring, O., Benitez Sanchez, S., Pasha, S. et al. Potential Role for Observation in Small Solid Pseudopapillary Neoplasm (SPN). Ann Surg Oncol 30, 5105–5112 (2023). https://doi.org/10.1245/s10434-023-13496-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-13496-2