Abstract
Background
Pancreatic cancer (PC) has an extremely high mortality rate, where obstructive jaundice due to cholestasis is a classic symptom. Conjugated bile acids (CBAs) such as taurocholic acid (TCA) have been reported to activate both the ERK1/2 and AKT signaling pathways via S1P receptor 2 (S1PR2) and promote growth of cholangiocarcinoma. Thus, we hypothesize that CBAs, which accumulate in cholestasis, accelerate PC progression via S1PR2.
Methods
Murine Panc02-luc and human AsPC-1, MIA PaCa2, and BxPC-3 cells were treated with TCA, S1PR2 agonist CYM5520, S1PR2 antagonist JTE-013, sphingosine-1-phosphate (S1P), and functional S1P receptor antagonist (except S1PR2) FTY720. Bile duct ligation (BDL) was performed on liver implantation or intraperitoneal injection of Panc02-luc cells.
Results
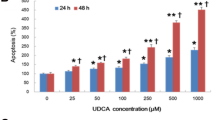
Panc02-luc and AsPC-1 cells predominantly expressed S1PR2, and their growth and migration were stimulated by TCA or CYM5520 in dose-dependent manner, which was blocked by JTE-013. This finding was not seen in PC cell lines expressing other S1P receptors than S1PR2. Panc02-luc growth stimulation by S1P was not blocked by FTY720. BDL significantly increased PC liver metastasis compared with sham. PC peritoneal carcinomatosis was significantly worsened by BDL, confirmed by number of nodules, tumor weight, bioluminescence, Ki-67 stain, ascites, and worse survival compared with sham. CYM5520 significantly worsened PC carcinomatosis, whereas treatment with anti-S1P antibody or FTY720 also worsened progression.
Conclusions
CBAs accelerated growth of S1PR2 predominant PC both in vitro and in vivo. This finding implicates S1PR2 as a potential therapeutic target in metastatic S1PR2 predominant pancreatic cancer.







Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. https://doi.org/10.3322/caac.21590.
Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378(9791):607–20. https://doi.org/10.1016/S0140-6736(10)62307-0.
van der Gaag NA, Kloek JJ, de Castro SM, Busch OR, van Gulik TM, Gouma DJ. Preoperative biliary drainage in patients with obstructive jaundice: history and current status. J Gastrointest Surg. 2009;13(4):814–20. https://doi.org/10.1007/s11605-008-0618-4.
Nagahashi M, Takabe K, Liu R, et al. Conjugated bile acid-activated S1P receptor 2 is a key regulator of sphingosine kinase 2 and hepatic gene expression. Hepatology. 2015;61(4):1216–26. https://doi.org/10.1002/hep.27592.
Park MA, Zhang G, Norris J, et al. Regulation of autophagy by ceramide-CD95-PERK signaling. Autophagy. 2008;4(7):929–31. https://doi.org/10.4161/auto.6732.
Studer E, Zhou X, Zhao R, et al. Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes. Hepatology. 2012;55(1):267–76. https://doi.org/10.1002/hep.24681.
Yang H, Li TW, Peng J, et al. A mouse model of cholestasis-associated cholangiocarcinoma and transcription factors involved in progression. Gastroenterology. 2011;141(1):378–88. https://doi.org/10.1053/j.gastro.2011.03.044.
Labib PL, Goodchild G, Pereira SP. Molecular pathogenesis of cholangiocarcinoma. BMC Cancer. 2019;19(1):185. https://doi.org/10.1186/s12885-019-5391-0.
Bernstein H, Bernstein C, Payne CM, Dvorak K. Bile acids as endogenous etiologic agents in gastrointestinal cancer. World J Gastroenterol. 2009;15(27):3329–40. https://doi.org/10.3748/wjg.15.3329.
Dai J, Wang H, Shi Y, Dong Y, Zhang Y, Wang J. Impact of bile acids on the growth of human cholangiocarcinoma via FXR. J Hematol Oncol. 2011;4:41. https://doi.org/10.1186/1756-8722-4-41.
Maroni L, Alpini G, Marzioni M. Cholangiocarcinoma development: the resurgence of bile acids. Hepatology. 2014;60(3):795–7. https://doi.org/10.1002/hep.27223.
Liu R, Zhao R, Zhou X, et al. Conjugated bile acids promote cholangiocarcinoma cell invasive growth through activation of sphingosine 1-phosphate receptor 2. Hepatology. 2014;60(3):908–18. https://doi.org/10.1002/hep.27085.
Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60(2):181–95. https://doi.org/10.1124/pr.107.07113.
Takabe K, Spiegel S. Export of sphingosine-1-phosphate and cancer progression. J Lipid Res. 2014;55(9):1839–46. https://doi.org/10.1194/jlr.R046656.
Sabbadini RA. Sphingosine-1-phosphate antibodies as potential agents in the treatment of cancer and age-related macular degeneration. Br J Pharmacol. 2011;162(6):1225–38. https://doi.org/10.1111/j.1476-5381.2010.01118.x.
Liang J, Nagahashi M, Kim EY, et al. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell. 2013;23(1):107–20. https://doi.org/10.1016/j.ccr.2012.11.013.
Aoyagi T, Nagahashi M, Yamada A, Takabe K. The role of sphingosine-1-phosphate in breast cancer tumor-induced lymphangiogenesis. Lymphat Res Biol. 2012;10(3):97–106. https://doi.org/10.1089/lrb.2012.0010.
Huang WC, Nagahashi M, Terracina KP, Takabe K. Emerging role of sphingosine-1-phosphate in inflammation, cancer, and lymphangiogenesis. Biomolecules. 2013. https://doi.org/10.3390/biom3030408.
Nagahashi M, Ramachandran S, Kim EY, et al. Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by stimulating angiogenesis and lymphangiogenesis. Cancer Res. 2012;72(3):726–35. https://doi.org/10.1158/0008-5472.CAN-11-2167.
Takabe K, Yamada A, Rashid OM, et al. Twofer anti-vascular therapy targeting sphingosine-1-phosphate for breast cancer. Gland Surg. 2012;1(2):80–3. https://doi.org/10.3978/j.issn.2227-684X.2012.07.01.
Bi Y, Li J, Ji B, et al. Sphingosine-1-phosphate mediates a reciprocal signaling pathway between stellate cells and cancer cells that promotes pancreatic cancer growth. Am J Pathol. 2014;184(10):2791–802. https://doi.org/10.1016/j.ajpath.2014.06.023.
Nagahashi M, Yuza K, Hirose Y, et al. The roles of bile acids and sphingosine-1-phosphate signaling in the hepatobiliary diseases. J Lipid Res. 2016;57(9):1636–43. https://doi.org/10.1194/jlr.R069286.
Little EC, Wang C, Watson PM, Watson DK, Cole DJ, Camp ER. Novel immunocompetent murine models representing advanced local and metastatic pancreatic cancer. J Surg Res. 2012;176(2):359–66. https://doi.org/10.1016/j.jss.2011.10.025.
Satsu H, Schaeffer MT, Guerrero M, et al. A sphingosine 1-phosphate receptor 2 selective allosteric agonist. Bioorg Med Chem. 2013;21(17):5373–82. https://doi.org/10.1016/j.bmc.2013.06.012.
Sun A, Hou L, Prugpichailers T, et al. Firefly luciferase-based dynamic bioluminescence imaging: a noninvasive technique to assess tumor angiogenesis. Neurosurgery. 2010;66(4):751–7. https://doi.org/10.1227/01.NEU.0000367452.37534.B1.
Aoki H, Aoki M, Yang J, et al. Murine model of long-term obstructive jaundice. J Surg Res. 2016;206(1):118–25. https://doi.org/10.1016/j.jss.2016.07.020.
Aoki H, Aoki M, Katsuta E, et al. Host sphingosine kinase 1 worsens pancreatic cancer peritoneal carcinomatosis. J Surg Res. 2016;205(2):510–7. https://doi.org/10.1016/j.jss.2016.05.034.
Liu R, Li X, Qiang X, et al. Taurocholate induces cyclooxygenase-2 expression via the sphingosine 1-phosphate receptor 2 in a human cholangiocarcinoma cell line. J Biol Chem. 2015;290(52):30988–1002. https://doi.org/10.1074/jbc.M115.668277.
Chen Z, Wu Y, Wang B, et al. Intrahepatic cholestasis induced by α-naphthylisothiocyanate can cause gut-liver axis disorders. Environ Toxicol Pharmacol. 2021;86:103672. https://doi.org/10.1016/j.etap.2021.103672.
Berntsen NL, Fosby B, Valestrand L, et al. Establishment of a surgical bile duct injection technique giving direct access to the bile ducts for studies of the murine biliary tree. Am J Physiol Gastrointest Liver Physiol. 2018;314(3):G349-g359. https://doi.org/10.1152/ajpgi.00124.2017.
Katsuta E, DeMasi SC, Terracina KP, et al. Modified breast cancer model for preclinical immunotherapy studies. J Surg Res. 2016;204(2):467–74. https://doi.org/10.1016/j.jss.2016.06.003.
Katsuta E, Oshi M, Rashid OM, Takabe K. Generating a murine orthotopic metastatic breast cancer model and performing murine radical mastectomy. J Vis Exp. 2018. https://doi.org/10.3791/57849.
Katsuta E, Rashid OM, Takabe K. Murine breast cancer mastectomy model that predicts patient outcomes for drug development. J Surg Res. 2017;219:310–8. https://doi.org/10.1016/j.jss.2017.06.048.
Katsuta E, Rashid OM, Takabe K. Clinical relevance of tumor microenvironment: immune cells, vessels, and mouse models. Hum Cell. 2020;33(4):930–7. https://doi.org/10.1007/s13577-020-00380-4.
Kawaguchi T, Foster BA, Young J, Takabe K. Current update of patient-derived xenograft model for translational breast cancer research. J Mammary Gland Biol Neoplasia. 2017;22(2):131–9. https://doi.org/10.1007/s10911-017-9378-7.
Okano M, Oshi M, Butash A, et al. Orthotopic implantation achieves better engraftment and faster growth than subcutaneous implantation in breast cancer patient-derived xenografts. J Mammary Gland Biol Neoplasia. 2020;25(1):27–36. https://doi.org/10.1007/s10911-020-09442-7.
Oshi M, Okano M, Maiti A, et al. Novel breast cancer brain metastasis patient-derived orthotopic xenograft model for preclinical studies. Cancers (Basel). 2020. https://doi.org/10.3390/cancers12020444.
Rashid OM, Nagahashi M, Ramachandran S, et al. An improved syngeneic orthotopic murine model of human breast cancer progression. Breast Cancer Res Treat. 2014;147(3):501–12. https://doi.org/10.1007/s10549-014-3118-0.
Rashid OM, Nagahashi M, Ramachandran S, et al. Is tail vein injection a relevant breast cancer lung metastasis model? J Thorac Dis. 2013;5(4):385–92. https://doi.org/10.3978/j.issn.2072-1439.2013.06.17.
Rashid OM, Takabe K. Animal models for exploring the pharmacokinetics of breast cancer therapies. Expert Opin Drug Metab Toxicol. 2015;11(2):221–30. https://doi.org/10.1517/17425255.2015.983073.
Terracina KP, Aoyagi T, Huang WC, et al. Development of a metastatic murine colon cancer model. J Surg Res. 2015;199(1):106–14. https://doi.org/10.1016/j.jss.2015.04.030.
Ikarashi M, Tsuchida J, Nagahashi M, et al. Plasma sphingosine-1-phosphate levels are associated with progression of estrogen receptor-positive breast cancer. Int J Mol Sci. 2021. https://doi.org/10.3390/ijms222413367.
Miura K, Nagahashi M, Prasoon P, et al. Dysregulation of sphingolipid metabolic enzymes leads to high levels of sphingosine-1-phosphate and ceramide in human hepatocellular carcinoma. Hepatol Res. 2021;51(5):614–26. https://doi.org/10.1111/hepr.13625.
Nagahashi M, Abe M, Sakimura K, Takabe K, Wakai T. The role of sphingosine-1-phosphate in inflammation and cancer progression. Cancer Sci. 2018;109(12):3671–8. https://doi.org/10.1111/cas.13802.
Oshi M, Newman S, Tokumaru Y, et al. Intra-tumoral angiogenesis is associated with inflammation, immune reaction and metastatic recurrence in breast cancer. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21186708.
Satyananda V, Oshi M, Tokumaru Y, et al. Sphingosine 1-phosphate (S1P) produced by sphingosine kinase 1 (SphK1) and exported via ABCC1 is related to hepatocellular carcinoma (HCC) progression. Am J Cancer Res. 2021;11(9):4394–407.
Tsuchida J, Nagahashi M, Nakajima M, et al. Sphingosine kinase 1 is associated with immune cell-related gene expressions in human breast cancer. J Surg Res. 2020;256:645–56. https://doi.org/10.1016/j.jss.2020.06.057.
Wang P, Yuan Y, Lin W, Zhong H, Xu K, Qi X. Roles of sphingosine-1-phosphate signaling in cancer. Cancer Cell Int. 2019;19:295. https://doi.org/10.1186/s12935-019-1014-8.
Gupta P, Taiyab A, Hussain A, Alajmi MF, Islam A, Hassan MI. Targeting the sphingosine kinase/sphingosine-1-phosphate signaling axis in drug discovery for cancer therapy. Cancers (Basel). 2021. https://doi.org/10.3390/cancers13081898.
Visentin B, Vekich JA, Sibbald BJ, et al. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell. 2006;9(3):225–38. https://doi.org/10.1016/j.ccr.2006.02.023.
Tong S, Chen SC, Xu KY, Fang B, Wang SH, Wang JJ. 14–3-3zeta promotes esophageal squamous cell carcinoma invasion by repressing S1PR2 protein expression through NF-kappaB signaling. Arch Biochem Biophys. 2018;643:7–13. https://doi.org/10.1016/j.abb.2018.02.009.
Pal SK, Drabkin HA, Reeves JA, et al. A phase 2 study of the sphingosine-1-phosphate antibody sonepcizumab in patients with metastatic renal cell carcinoma. Cancer. 2017;123(4):576–82. https://doi.org/10.1002/cncr.30393.
Wang Y, Aoki H, Yang J, et al. The role of sphingosine 1-phosphate receptor 2 in bile-acid-induced cholangiocyte proliferation and cholestasis-induced liver injury in mice. Hepatology. 2017;65(6):2005–18. https://doi.org/10.1002/hep.29076.
Brinkmann V, Billich A, Baumruker T, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010;9(11):883–97. https://doi.org/10.1038/nrd3248.
Azuma H, Takahara S, Horie S, Muto S, Otsuki Y, Katsuoka Y. Induction of apoptosis in human bladder cancer cells in vitro and in vivo caused by FTY720 treatment. J Urol. 2003;169(6):2372–7. https://doi.org/10.1097/01.ju.0000064938.32318.91.
Bai LY, Chiu CF, Chiu SJ, Chu PC, Weng JR. FTY720 induces autophagy-associated apoptosis in human oral squamous carcinoma cells, in part, through a reactive oxygen species/mcl-1-dependent mechanism. Sci Rep. 2017;7(1):5600. https://doi.org/10.1038/s41598-017-06047-9.
Lucas da Silva LB, Ribeiro DA, Cury PM, Cordeiro JA, Bueno V. FTY treatment in experimentally urethane-induced lung tumors. J Exp Ther Oncol. 2008;7(1):9–15.
Acknowledgement
None.
Funding
This research was supported by National Institutes of Health, USA Grant Nos. R01CA160688, R37CA248018, R01CA250412, R01CA251545, and R01EB029596, as well as US Department of Defense BCRP Grant Nos. W81XWH-19-1-0674 and W81XWH-19-1-0111 to K.T.; VA Merit Award I01BX004033, Research Career Scientist Award (IK6BX004477), VA ShEEP Grant (1 IS1 BX004777), National Institutes of Health Grant R01 DK104893 and R01DK-057543 to H.Z.; National Cancer Institute, cancer center support Grant P30CA016056 to Roswell Park Comprehensive Cancer Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors have no potential conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sarkar, J., Aoki, H., Wu, R. et al. Conjugated Bile Acids Accelerate Progression of Pancreatic Cancer Metastasis via S1PR2 Signaling in Cholestasis. Ann Surg Oncol 30, 1630–1641 (2023). https://doi.org/10.1245/s10434-022-12806-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-12806-4