Abstract
Background
The 8th edition of the TNM stage classification of lung cancer was developed based on an evaluation of the 5-year prognosis using an international database. Since recurrence after 5 years postoperatively is known to develop, the applicability of the stage classification beyond 5 years after treatment needs to be evaluated.
Patients and Methods
Postoperative prognosis and prognostic indicators were analyzed using data for 648 patients of pathological stage IA adenocarcinoma, who underwent complete resection between 2007 and 2012.
Results
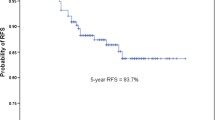
The median age was 66 years (interquartile range 60–73 years), and the median follow-up duration was 100 months (interquartile range 70–116 months). Overall survival probabilities for pathological stage IA1, IA2, and IA3 patients were 100%, 96.3%, and 91.5% at 5 postoperative years, and 94.2%, 89.8%, and 83.5% at 10 postoperative years, respectively (IA1 vs IA2: p = 0.05; IA2 vs IA3: p = 0.05). Multivariate analysis for overall survival of patients who survived without recurrence for 5 postoperative years revealed that age (hazard ratio 3.21, p = 0.02) was the only factor that was significantly associated with long-term survival. Stage classification (IA1, IA2, or IA3) was not an associated factor. The incidence of secondary primary lung cancer continued to increase, resulting in an estimated probability of 8.6% at 10 postoperative years.
Conclusions
For patients who survived without recurrence for 5 postoperative years, age, not stage classification, was associated with survival thereafter. The long-term follow-up strategy does not need to be modified according to the stage classification, and screening for secondary primary lung cancer should be considered.



Similar content being viewed by others
References
Rami-Porta R, Bolejack V, Giroux DJ, et al. The IASLC lung cancer staging project: the new database to inform the eighth edition of the TNM classification of lung cancer. J Thorac Oncol. 2014;9(11):1618–24.
Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for the revisions of the t descriptors in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol. 2015;10(7):990–1003.
Asamura H, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: proposals for the revision of the N Descriptors in the forthcoming 8th edition of the TNM classification for lung cancer. J Thorac Oncol. 2015;10(12):1675–84.
Eberhardt WE, Mitchell A, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the M descriptors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol. 2015;10(11):1515–22.
Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39–51.
Takano T, Ohe Y, Tsuta K, et al. Epidermal growth factor receptor mutation detection using high-resolution melting analysis predicts outcomes in patients with advanced non small cell lung cancer treated with gefitinib. Clin Cancer Res. 2007;13(18 Pt 1):5385–90.
Suzuki K, Saji H, Aokage K, et al. Comparison of pulmonary segmentectomy and lobectomy: safety results of a randomized trial. J Thorac Cardiovasc Surg. 2019;158(3):895–907.
Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol. 2010;40(3):271–4.
Felip E, Ardizzoni A, Ciuleanu T, et al. CheckMate 171: a phase 2 trial of nivolumab in patients with previously treated advanced squamous non-small cell lung cancer, including ECOG PS 2 and elderly populations. Eur J Cancer. 2020;127:160–72.
Berry MF, Hanna J, Tong BC, et al. Risk factors for morbidity after lobectomy for lung cancer in elderly patients. Ann Thorac Surg. 2009;88(4):1093–9.
Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman BJ, Senan S. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol. 2010;28(35):5153–9.
Maeda R, Yoshida J, Hishida T, et al. Late recurrence of non-small cell lung cancer more than 5 years after complete resection: incidence and clinical implications in patient follow-up. Chest. 2010;138(1):145–50.
Maeda R, Yoshida J, Ishii G, et al. Long-term outcome and late recurrence in patients with completely resected stage IA non-small cell lung cancer. J Thorac Oncol. 2010;5(8):1246–50.
Pasini F, Pelosi G, Valduga F, et al. Late events and clinical prognostic factors in stage I non small cell lung cancer. Lung Cancer. 2002;37(2):171–7.
Ito H, Suzuki K, Mizutani T, et al. Long-term survival outcome after lobectomy in patients with clinical T1 N0 lung cancer. J Thorac Cardiovasc Surg. 2020. https://doi.org/10.1016/j.jtcvs.2019.12.072.
Kunitoh H, Tsuboi M, Wakabayashi M, et al. A phase III study of adjuvant chemotherapy in patients with completely resected, node-negative non–small cell lung cancer (JCOG 0707). JTCVS Open. 2020;4:90–102.
Ferguson MK, Watson S, Johnson E, Vigneswaran WT. Predicted postoperative lung function is associated with all-cause long-term mortality after major lung resection for cancer. Eur J Cardiothorac Surg. 2014;45(4):660–4.
Arriagada R, Dunant A, Pignon JP, et al. Long-term results of the international adjuvant lung cancer trial evaluating adjuvant Cisplatin-based chemotherapy in resected lung cancer. J Clin Oncol. 2010;28(1):35–42.
Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350(4):351–60.
Hu ZG, Li WX, Ruan YS, Zeng FJ. Incidence trends and risk prediction nomogram of metachronous second primary lung cancer in lung cancer survivors. PLoS ONE. 2018;13(12):e0209002.
Thakur MK, Ruterbusch JJ, Schwartz AG, Gadgeel SM, Beebe-Dimmer JL, Wozniak AJ. Risk of second lung cancer in patients with previously treated lung cancer: analysis of Surveillance, Epidemiology, and End Results (SEER) data. J Thorac Oncol. 2018;13(1):46–53.
Han SS, Rivera GA, Tammemagi MC, et al. Risk stratification for second primary lung cancer. J Clin Oncol. 2017;35(25):2893–9.
Hanna WC. Pros: long-term CT scan follow-up should be the standard of care in patients who are curatively treated for an early-stage non-small cell lung cancer. Transl Lung Cancer Res. 2015;4(4):476–8.
Network NCC. NCCN Clinical guidelines in oncology: non-small cell lung cancer. 2020.
Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e278S-e313S.
Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):1–21.
Acknowledgment
We thank members of the Division of Biostatistics at the National Cancer Center for offering fruitful opinions and suggestions on the statistical methods.
Funding
This work was supported in part by KAKENHI grants from the Ministry of Education, Culture, Sports, Science, and Technology [Grant Numbers 16KT0197 (Kouya Shiraishi) and 20H00545 (Takashi Kohno)] and a grant from the Japan Agency for Medical Research and Development [Grant Number 19ck0106323h003 (Shun-ichi Watanabe)].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Yasushi Yatabe has received personal fees outside of this work from MSD, Chugai Pharmaceutical, Astra Zeneca, Pfizer Japan, Roche/Ventana, Agilent/Dako, Thermo Fisher Scientific, Archer CD, Novartis Pharma, Eli Lilly Japan, Amgen, Merck Biopharma, Sysmex, Bayer, and Daiichi Sankyo. Takashi Kohno has received personal fees outside of this work from Chugai Pharmaceutical, Eli Lilly Japan, and Sysmex Corporation.
Ethical Approval
The study protocol was approved by the Medical Research Ethics Committee of the National Cancer Center (IRB approval no. 2015-289), and all experiments were conducted in accordance with the Declaration of Helsinki. The requirement for informed consent was waived by the committee since our study was a retrospective review of patient records.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yotsukura, M., Muraoka, Y., Yoshida, Y. et al. Long-Term Prognosis and Prognostic Indicators of Stage IA Lung Adenocarcinoma. Ann Surg Oncol 30, 851–858 (2023). https://doi.org/10.1245/s10434-022-12621-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-12621-x