Abstract
Background
We wanted to investigate the association between circulating tumor DNA (ctDNA) detection at baseline, during and after neoadjuvant treatment, after surgery, and recurrence, in patients with nonmetastatic cancer.
Patients and Methods
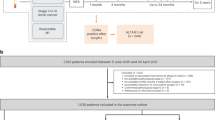
In this systematic review and meta-analysis, we included studies that investigated patients undergoing neoadjuvant treatment for nonmetastatic cancer and provided recurrence indices stratified for ctDNA status at the following timepoints: baseline, during treatment, posttreatment, and postsurgery. Study quality was reported with the Newcastle–Ottawa scale, REMARK checklist, and GRADE approach. PubMed, Embase, Cochrane Library, and Web of Science were our data sources (inception to 3 June 2021). The main outcome was risk of recurrence.
Results
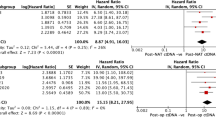
We identified ten studies including 727 patients with rectal, breast, gastric, and bladder cancer. All studies reported posttreatment ctDNA analysis, while seven, four, and six reported baseline, during treatment, and postsurgery ctDNA analysis, respectively. ctDNA detection was associated to recurrence across all timepoints [baseline: risk ratio (RR) 2.86, 95% confidence interval (CI) 1.33–6.14, during treatment: RR 3.81, 95% CI 2.09–6.92, posttreatment: RR 4.29, 95% CI 2.79–6.60, postsurgery: RR 8.03, 95% CI 3.16–20.43]. Heterogeneity was low to moderate.
Conclusions
This meta-analysis of observational studies found that ctDNA detection in patients undergoing neoadjuvant treatment for nonmetastatic cancer was associated with recurrence. A stronger association was evident in posttreatment and postsurgery timepoints. However, some studies reported low negative predictive value (NPV) of pathological complete response, showing that ctDNA-detection-guided escalation and de-escalation studies following neoadjuvant treatment regimens are needed before its role as a treatment guidance can be affirmed.


Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. https://doi.org/10.3322/caac.21551.
Kapiteijn E, Marijnen CAM, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345(9):638–46. https://doi.org/10.1056/nejmoa010580.
Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349(9):859–66. https://doi.org/10.1056/nejmoa022148.
van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–84. https://doi.org/10.1056/nejmoa1112088.
Burdett S. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet. 2014;383(9928):1561–71. https://doi.org/10.1016/S0140-6736(13)62159-5.
Provencio M, Nadal E, Insa A, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21(11):1413–22. https://doi.org/10.1016/S1470-2045(20)30453-8.
Bahadoer RR, Dijkstra EA, van Etten B, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):29–42. https://doi.org/10.1016/S1470-2045(20)30555-6.
Dossa F, Chesney TR, Acuna SA, Baxter NN. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2(7):501–13. https://doi.org/10.1016/S2468-1253(17)30074-2.
Suppiah A, Hunter IA, Cowley J, et al. Magnetic resonance imaging accuracy in assessing tumour down-staging following chemoradiation in rectal cancer. Color Dis. 2009;11(3):249–53. https://doi.org/10.1111/j.1463-1318.2008.01593.x.
Beets-Tan RGH, Beets GL. MRI for assessing and predicting response to neoadjuvant treatment in rectal cancer. Nat Rev Gastroenterol Hepatol. 2014;11(8):480–8. https://doi.org/10.1038/nrgastro.2014.41.
Dossa F, Acuna SA, Rickles AS, et al. Association between adjuvant chemotherapy and overall survival in patients with rectal cancer and pathological complete response after neoadjuvant chemotherapy and resection. JAMA Oncol. 2018;4(7):930–7. https://doi.org/10.1001/jamaoncol.2017.5597.
Reinert T, Henriksen TV, Christensen E, et al. Analysis of plasma cell-free DNA by ultradeep sequencing in patients with stages i to III colorectal cancer. JAMA Oncol. 2019;5(8):1124–31. https://doi.org/10.1001/jamaoncol.2019.0528.
Christensen E, Birkenkamp-Demtröder K, Sethi H, et al. Early detection of metastatic relapse and monitoring of therapeutic efficacy by ultra-deep sequencing of plasma cell-free DNA in patients with urothelial bladder carcinoma. J Clin Oncol. 2019;37(18):1547–57. https://doi.org/10.1200/JCO.18.02052.
Schøler LV, Reinert T, Ørntoft MBW, et al. Clinical implications of monitoring circulating Tumor DNA in patients with colorectal cancer. Clin Cancer Res. 2017;23(18):5437–45. https://doi.org/10.1158/1078-0432.CCR-17-0510.
Reinert T, Schøler LV, Thomsen R, et al. Analysis of circulating tumour DNA to monitor disease burden following colorectal cancer surgery. Gut. 2016;65(4):625–34. https://doi.org/10.1136/gutjnl-2014-308859.
Tie J, Wang Y, Cohen J, et al. Circulating tumor DNA dynamics and recurrence risk in patients undergoing curative intent resection of colorectal cancer liver metastases: a prospective cohort study. PLoS Med. 2021. https://doi.org/10.1371/journal.pmed.1003620.
Chen G, Peng J, Xiao Q, et al. Postoperative circulating tumor DNA as markers of recurrence risk in stages II to III colorectal cancer. J Hematol Oncol. 2021;14(1):80. https://doi.org/10.1186/s13045-021-01089-z.
Tarazona N, Gimeno-Valiente F, Gambardella V, et al. Targeted next-generation sequencing of circulating-tumor DNA for tracking minimal residual disease in localized colon cancer. Ann Oncol. 2019;30(11):1804–12. https://doi.org/10.1093/annonc/mdz390.
Nors J, Henriksen TV, Gotschalck KA, et al. IMPROVE-IT2: implementing noninvasive circulating tumor DNA analysis to optimize the operative and postoperative treatment for patients with colorectal cancer—intervention trial 2. Study protocol. Acta Oncol (Madr). 2020;59(3):336–41. https://doi.org/10.1080/0284186X.2019.1711170.
Taniguchi H, Nakamura Y, Kotani D, et al. CIRCULATE-Japan: Circulating tumor DNA–guided adaptive platform trials to refine adjuvant therapy for colorectal cancer. Cancer Sci. 2021;112(7):2915–20. https://doi.org/10.1111/cas.14926.
Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015: elaboration and explanation. BMJ. 2015;349:g7647. https://doi.org/10.1136/bmj.g7647.
Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. J Am Med Assoc. 2000;283(15):2008–12. https://doi.org/10.1001/jama.283.15.2008.
Wells G, Shea B, O’Connell D, Peterson J. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa, ON: Ottawa Hospital Research Institute. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Published 2000.
Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting recommendations for tumor marker prognostic studies (REMARK): explanation and elaboration. BMC Med. 2012;10:51. https://doi.org/10.1186/1741-7015-10-51.
Iorio A, Spencer FA, Falavigna M, et al. Use of GRADE for assessment of evidence about prognosis: Rating confidence in estimates of event rates in broad categories of patients. BMJ. 2015;350:h870. https://doi.org/10.1136/bmj.h870.
Huguet A, Hayden JA, Stinson J, et al. Judging the quality of evidence in reviews of prognostic factor research: adapting the GRADE framework. Syst Rev. 2013;2(1):71. https://doi.org/10.1186/2046-4053-2-71.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315(7109):629–34. https://doi.org/10.1136/bmj.315.7109.629.
Vidal J, Casadevall D, Bellosillo B, et al. Clinical impact of presurgery circulating tumor DNA after total neoadjuvant treatment in locally advanced rectal cancer: a biomarker study from the GEMCAD 1402 trial. Clin Cancer Res. 2021;27(10):2890–8. https://doi.org/10.1158/1078-0432.CCR-20-4769.
Tie J, Cohen JD, Wang Y, et al. Serial circulating tumour DNA analysis during multimodality treatment of locally advanced rectal cancer: a prospective biomarker study. Gut. 2019;68(4):663–71. https://doi.org/10.1136/gutjnl-2017-315852.
Khakoo S, Carter PD, Brown G, et al. MRI tumor regression grade and circulating tumor DNA as complementary tools to assess response and guide therapy adaptation in rectal cancer. Clin Cancer Res. 2020;26(1):183–92. https://doi.org/10.1158/1078-0432.CCR-19-1996.
Murahashi S, Akiyoshi T, Sano T, et al. Serial circulating tumour DNA analysis for locally advanced rectal cancer treated with preoperative therapy: prediction of pathological response and postoperative recurrence. Br J Cancer. 2020;123(5):803–10. https://doi.org/10.1038/s41416-020-0941-4.
Zhou J, Wang C, Lin G, et al. Serial circulating tumor DNA in predicting and monitoring the effect of neoadjuvant chemoradiotherapy in patients with rectal cancer: a prospective multicenter study. Clin Cancer Res. 2021;27(1):301–10. https://doi.org/10.1158/1078-0432.CCR-20-2299.
Magbanua MJM, Swigart LB, Wu HT, et al. Circulating tumor DNA in neoadjuvant-treated breast cancer reflects response and survival. Ann Oncol. 2021;32(2):229–39. https://doi.org/10.1016/j.annonc.2020.11.007.
Cavallone L, Aguilar-Mahecha A, Lafleur J, et al. Prognostic and predictive value of circulating tumor DNA during neoadjuvant chemotherapy for triple negative breast cancer. Sci Rep. 2020. https://doi.org/10.1038/s41598-020-71236-y.
Ortolan E, Appierto V, Silvestri M, et al. Blood-based genomics of triple-negative breast cancer progression in patients treated with neoadjuvant chemotherapy. ESMO Open. 2021;6(2):100086. https://doi.org/10.1016/j.esmoop.2021.100086.
Leal A, van Grieken NCT, Palsgrove DN, et al. White blood cell and cell-free DNA analyses for detection of residual disease in gastric cancer. Nat Commun. 2020. https://doi.org/10.1038/s41467-020-14310-3.
Ryan R, Gibbons D, Hyland JMP, et al. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology. 2005;47(2):141–6. https://doi.org/10.1111/j.1365-2559.2005.02176.x.
Jang JK, Choi SH, Park SH, et al. MR tumor regression grade for pathological complete response in rectal cancer post neoadjuvant chemoradiotherapy: a systematic review and meta-analysis for accuracy. Eur Radiol. 2020;30(4):2312–23. https://doi.org/10.1007/s00330-019-06565-2.
Chalabi M, Fanchi LF, Dijkstra KK, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. 2020;26(4):566–76. https://doi.org/10.1038/s41591-020-0805-8.
Patel A, Spychalski P, Corrao G, et al. Neoadjuvant short-course radiotherapy with consolidation chemotherapy for locally advanced rectal cancer: a systematic review and meta-analysis. Acta Oncol (Madr). 2021;60(10):1308–16. https://doi.org/10.1080/0284186X.2021.1953137.
Tie J, Cohen JD, Wang Y, et al. Circulating tumor dna analyses as markers of recurrence risk and benefit of adjuvant therapy for stage III colon cancer. JAMA Oncol. 2019;5(12):1710–7. https://doi.org/10.1001/jamaoncol.2019.3616.
Dasari A, Morris VK, Allegra CJ, et al. ctDNA applications and integration in colorectal cancer: an NCI Colon and Rectal-Anal Task Forces whitepaper. Nat Rev Clin Oncol. 2020;17(12):757–70. https://doi.org/10.1038/s41571-020-0392-0.
Zhang L, Zhang Y, Chang L, et al. Intratumor heterogeneity comparison among different subtypes of non-small-cell lung cancer through multi-region tissue and matched ctDNA sequencing. Mol Cancer. 2019;18(1):7. https://doi.org/10.1186/s12943-019-0939-9.
Baumgartner JM, Riviere P, Lanman RB, et al. Prognostic utility of pre- and postoperative circulating tumor dna liquid biopsies in patients with peritoneal metastases. Ann Surg Oncol. 2020;27(9):3259–67. https://doi.org/10.1245/s10434-020-08331-x.
Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6(224):224ra24. https://doi.org/10.1126/scitranslmed.3007094.
Cabel L, Proudhon C, Romano E, et al. Clinical potential of circulating tumour DNA in patients receiving anticancer immunotherapy. Nat Rev Clin Oncol. 2018;15(10):639–50. https://doi.org/10.1038/s41571-018-0074-3.
von Minckwitz G, Huang C-S, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–28. https://doi.org/10.1056/nejmoa1814017.
Author information
Authors and Affiliations
Contributions
Conceptualization: MG, IG; methodology: MG; software: MG; validation: NA; formal analysis: MG; investigation: MG, IG, resources: MG, NH; data curation: MG, NH; writing: original draft: MG, IG, writing: review and editing: MG, IG, NH, CQ, CA; visualization: MG, supervision: IG: project administration: MG; funding acquisition: no funding received.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gögenur, M., Hadi, N.AH., Qvortrup, C. et al. ctDNA for Risk of Recurrence Assessment in Patients Treated with Neoadjuvant Treatment: A Systematic Review and Meta-analysis. Ann Surg Oncol 29, 8666–8674 (2022). https://doi.org/10.1245/s10434-022-12366-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-12366-7