Abstract
Background
One fourth of early-stage breast cancer cases become metastatic during the follow-up period. Limited metastasis is a metastatic disease condition in which the number of metastatic sites and the extent of the disease both are limited, and the disease is amenable to metastatic intervention. This prospective study aimed to evaluate intervention for limited metastases in the lung, liver, or both.
Methods
The study enrolled luminal A/B and/or human epidermal growth factor receptor 2 (HER2)-neu+ patients with operable lung and/or liver metastases in the follow-up assessment after completion of primary breast cancer treatment and patients with a diagnosis of metastasis after 2014. Demographic, clinical, tumor-specific, and metastasis detection-free interval (MDFI) data were collected. Bone metastasis in addition to lung and liver metastases also was included in the analysis. The patients were divided into two groups according to the method of treatment for metastases: systemic therapy alone (ST) group or intervention (IT) group.
Results
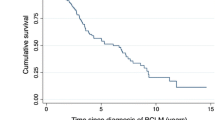
Until June 2020, 200 patients were enrolled in the study. The demographic data were similar between the two groups. The median follow-up time was 77 months (range 55–107 months) in the IT group (n = 119; 59.5%) and 57 months (range 39–84) in the ST-only group (n = 81; 40.5%). The median MDFI was 40 months (range 23–70 months) in the IT group, and 35 months (range 13–61 months) in the ST-only group (p = 0.47). The groups had similar surgeries for the primary tumor and axilla. Most of the patients had liver metastases (49.5%, n = 99), and 42% (n = 84) of the patients had lung metastases. Both lung and liver metastases were found in 8.5% (n = 17) of the patients. The primary tumor was estrogen receptor/progesterone receptor-positive in 75% (n = 150) of the patients, and 32% (n = 64) of the patients had HER2-neu+ tumors. Metastatic-site resection was performed for 32% (n = 64) of the patients, and 27.5% (n = 55) of the patients underwent metastatic ablative interventions. In the Kaplan-Meier survival analysis, the hazard of death (HoD) was 56% lower in the IT group than in the ST-only group (hazard ratio [HR], 0.44; 95% confidence interval [CI] 0.26–0.72; p = 0.001). The HoD was lower in the IT group than in the ST-only group for the patients younger than 55 years (HR, 0.32; 95% CI 0.17–0.62; p = 0.0007). In the multivariable Cox regression model, HoD was significantly lower for the patients who underwent intervention for metastases and had an MDFI longer than 24 months, but their liver metastases doubled the risk of death compared with lung metastases.
Conclusion
Metastasis-directed interventions have reduced the risk of death for patients with limited lung/liver metastases who are amenable to interventions after completion of primary cancer treatment. For a select group of patients, such as those with luminal A/B or HER2-neu+ breast cancer who are younger than 55 years with limited metastases to the lung and liver or an MDFI longer than 24 months, surgical or ablative therapy for metastases should be considered and discussed on tumor boards.


Similar content being viewed by others
Change history
09 December 2022
A Correction to this paper has been published: https://doi.org/10.1245/s10434-022-12931-0
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Howlader N, Noone AM, Krapcho M et al (eds). SEER cancer statistics review. National Cancer Institute, Bethesda; 1975–2008. https://seer.cancer.gov/csr/1975_2008/.
Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;365:1687–717.
Ruiz A, van Hillegersberg R, Siesling S, et al. Surgical resection versus systemic therapy for breast cancer liver metastases: results of a European case-matched comparison. Eur J Cancer. 2018;95:1–10.
Soran A, Dogan L, Isık A, Ozbas et al. The effect of primary surgery in patients with stage IV breast cancer with bone metastasis only (protocol BOMET MF 14–01): a multi-center, registry study. Ann Surg Oncol. 2021;28:5048–57.
National Comprehensive Cancer Network. Breast cancer; National Comprehensive Cancer Network Version 3.2019; 2019.
Pagani O, Senkus E, Wood W, Colleoni M, Cufer T, Kyriakides S, et al. International guidelines for management of metastatic breast cancer: Can metastatic breast cancer be cured? J Natl Cancer Inst. 2010;102:456–63.
Soran A, Ozmen V, Ozbas S, et al. Randomized trial comparing resection of primary tumor with no surgery in stage IV breast cancer at presentation: protocol MF07-01. Ann Surg Oncol. 2018;25:3141–9.
Lane W, Thomas S, Blitzblau R, et al. Surgical resection of the primary tumor in women with de novo stage IV breast cancer: contemporary practice patterns and survival analysis. Ann Surg. 2019;269:537–44.
Cheung T, Chok K, Chan A, et al. Survival analysis of breast cancer liver metastasis treated by hepatectomy: a propensity score analysis for Chinese women in Hong Kong. Hepatobiliary Pancreat Dis Int. 2019;18:452–7.
McDonald ML, Deschamps C, Ilstrup DM, et al. Pulmonary resection for metastatic breast cancer. Ann Thorac Surg. 1994;58:1599–602.
Orlandi A, Pontolillo L, Mele C, et al. Liver metastasectomy for metastatic breast cancer patients; a single-institution retrospective analysis. J Pers Med. 2021;11:187.
Cardoso F, Costa A, Norton L, et al. 1st International consensus guidelines for advanced breast cancer (ABC 1). Breast. 2012;21:242–52.
Palma DA, Olson R, Harrow S, Gaede S, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol. 2020;38:2830–8.
Ruiz A, Sebagh M, WichertsD A, Castro-Benitez C, van Hillegersberg R, Paule B, et al. Long-term survival and cure model following liver resection for breast cancer metastases. Breast Cancer Res Treat. 2018;170:89–100.
Xie J, Xu Z. A population-based study on liver metastases in women with newly diagnosed breast cancer. Cancer Epidemiol Biomarkers Prev. 2018;28:283–92.
National Cancer Institute. SEER stat fact sheets: female breast cancer. 2015.
Sadot E, Lee S, Sofocleous C, et al. Hepatic resection or ablation for isolated breast cancer liver metastasis: a case-control study with comparison to medically treated patients. Ann Surg. 2016;264:147–54.
Mariani P, Servois V, De Rycke Y, et al. Liver metastases from breast cancer: surgical resection or not? A case-matched control study in highly selected patients. Eur J Surg Oncol. 2013;39:1377–83.
Millen JA, Alana Hofmann A, Mesquita-Neto JW, Rose J, Macedo FI. Evolving role of liver resection in selected patients with metastatic breast cancer. J Surg Res. 2021;259:363–71.
Wyld L, Gutteridge E, Pinder SE, James JJ, Chan SY, Cheung KL, et al. Prognostic factors for patients with hepatic metastases from breast cancer. Br J Cancer. 2003;89:284–90.
Yoo TG, Cranshaw I, Broom R. Systematic review of early and long-term outcome of liver resection for metastatic breast cancer: Is there a survival benefit? Breast. 2017;32:162–72.
Wen J, Ye F, Xie F, Liu D, Huang L, Fang C, Zhong S, Ren L. The role of surgical intervention for isolated breast cancer liver metastasis: results of case-control study with comparison to medical treatment. Cancer Med. 2020;9:4656–66.
Chun YS, Mizuno T, Cloyd JM, Ha MJ, Omichi K, Tzeng CD, et al. Hepatic resection for breast cancer liver metastases: impact of intrinsic subtypes. Eur J Surg Oncol. 2020;46:1588–95.
Ji L, Cheng L, Zhu X, Gao Y, Fan L, Wang Z. Risk and prognostic factors of breast cancer with liver metastases. BMC Cancer. 2021;21:238.
Orlandi A, Pontolillo L, Mele C, et al. Liver metastasectomy for metastatic breast cancer patients: a single-institution retrospective analysis. J Pers Med. 2021;11:187.
He X, Zhang Q, Feng Y, Li Z, Pan Q, Zhao Y, et al. Resection of liver metastases from breast cancer: a multicentre analysis. Clin Transl Oncol. 2020;22:512–21.
Pierga JY, Asselain B, Jouve M, et al. Effect of adjuvant chemotherapy on outcome in patients with metastatic breast carcinoma treated with first-line doxorubicin-containing chemotherapy. Cancer. 2001;91:1079–89.
Meimarakis G, Rüttinger D, Stemmler J, Crispin A, Weidenhagen R, Angele M, et al. Prolonged overall survival after pulmonary metastasectomy in patients with breast cancer. Ann Thorac Surg. 2013;95:1170–80.
Friedel G, Pastorino U, Ginsberg RJ, et al. Results of lung metastasectomy from breast cancer: prognostic criteria on the basis of 467 cases of the International Registry of Lung Metastases. Eur J Cardiothorac Surg. 2002;22:335–44.
Fan J, Chen D, Du H, et al. Prognostic factors for resection of isolated pulmonary metastases in breast cancer patients: a systematic review and meta-analysis. J Thorac Dis. 2015;7:1441–51.
Endoh M, Shiono S, Yamauchi Y, Mun M, Ikeda N, Hashimoto H, et al. Pulmonary metastasectomy for pulmonary metastasis of breast cancer has a limited prognostic impact: a multi-institutional retrospective analysis. J Thorac Dis. 2020;12:6552–62.
Barberi V, Pietragalla A, Franceschini G, Marazzi F, Paris I, Cognetti F, et al. Oligometastatic breast cancer: How to manage it? J Pers Med. 2021;11:532.
Xiao YB, Zhang B, Wu YL. Radiofrequency ablation versus hepatic resection for breast cancer liver metastasis: a systematic review and meta-analysis. J Zhejiang Univ Sci B. 2018;19:829–43.
Kent CL, McDuff SGR, Salama JK. Oligometastatic breast cancer: where are we now and where are we headed? A narrative review. Ann Palliat Med. 2021;10:5954–68.
Acknowledgment
The authors thank Ahmet Bilgehan Sahin, Alper Toker, Arife Simsek, Ayfer Kamali Polat, Erkan Kaba, Eyup Anil Balkan, Ferah Yıldız, Hale Caglar, Halil Ibrahim Yildiz, Hande Koksal, Havva Belma Kocer, Ibrahim Ali Ozemir, Levent Yeniay, Mehmet Velidedeoglu, Menekse Turna, Metin Altinkaya, Merdan Fayda, Musa Baris Aykan, Mustafa Umit Ugurlu, Neslihan Cabioglu, Niyazi Karaman, Nuran Bese, Osman Toktas, Sefa Ergun, Semra Demirli Atici, Turkkan Evrensel for contributing to this study by giving a case. They also thank Christine Burr, scientific writer from the University of Pittsburgh, for her assistance with language editing.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Disclosure
There are no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soran, A., Ozbas, S., Ozcinar, B. et al. Intervention for Hepatic and Pulmonary Metastases in Breast Cancer Patients: Prospective, Multi-institutional Registry Study–IMET, Protocol MF 14-02. Ann Surg Oncol 29, 6327–6336 (2022). https://doi.org/10.1245/s10434-022-12239-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-12239-z